Perrinet Q., Ghosh A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F., Roland T., De Waele V.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F., Roland T., De Waele V.
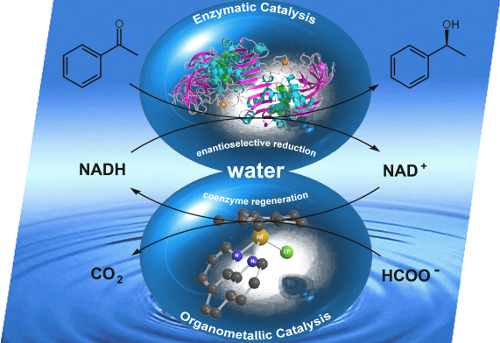
Unexpected Interference of the Triethanolamine Sacrificial Electron Donor with the Excited States of Molecular and Heterogenized Rhodium Bipyridine Photocatalysts Revealed by Femtosecond Transient Absorption Spectroscopy
JOURNAL OF PHYSICAL CHEMISTRY C, 2025, p.
Beucher R., Lacote E.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
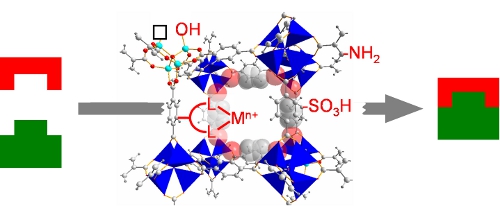
Molecular copper complexes embedded within porous polymer macroligands for the heterogeneously catalyzed aerobic oxidative formation of N-N bonds
NEW JOURNAL OF CHEMISTRY, 2024, p.
Riddhi R., Penas-Hidalgo F., Chen H.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Mellot-Draznieks C., Sole-Daura A.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Mellot-Draznieks C., Sole-Daura A.
Experimental and computational aspects of molecular frustrated Lewis pairs for CO2 hydrogenation: en route for heterogeneous systems?
CHEMICAL SOCIETY REVIEWS, 2024, p.
Ranscht A., Rigodanza F., Gobbato T., Crea I.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bonchio M.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bonchio M.
Combined Covalent and Supramolecular Polymerization to Reinforce Perylenebisimide Photosynthetic "Quantasomes"
CHEMISTRY-A EUROPEAN JOURNAL, 2024, p.
Samanta P.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
MOF-Supported Heterogeneous Catalysts for Hydroformylation Reactions: A Minireview
CHEMCATCHEM, 2024, p.
Newar R., Ghosh A., Riddhi R., Rajapaksha R., Samanta P., Wisser F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Molecular Rhodium Complex within N-Rich Porous Polymer Macroligand as Heterogeneous Catalyst for the Visible-Light Driven CO2 Photoreduction
ADVANCED ENERGY AND SUSTAINABILITY RESEARCH, 2023, p.
Samanta P., Beucher R., Riddhi R., Ranscht A., Wisser F.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
N-Heterocyclic carbene-based porous polymer macroligand for the Ni-catalyzed C-H arylation of benzothiophenes
CATALYSIS SCIENCE & TECHNOLOGY, 2023, 13(20), pp. 5825-5830
Rajapaksha R., Samanta P.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Heterogenization of molecular catalysts within porous solids: the case of Ni-catalyzed ethylene oligomerization from zeolites to metal-organic frameworks
CHEMICAL SOCIETY REVIEWS, 2023, p.
Jabbour R., Ashling C., Robinson T., Khan A., Wisser D., Berruyer P., Ghosh A., Ranscht A., Keen D., Brunner E.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bennett T., Mellot-Draznieks C., Lesage A., Wisser F.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bennett T., Mellot-Draznieks C., Lesage A., Wisser F.
Unravelling the Molecular Structure and Confining Environment of an Organometallic Catalyst Heterogenized within Amorphous Porous Polymers
ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 2023, p.
Favaro M., Yang J., Ditz D., Kucukkececi H., Alkhurisi M., Bergwinkl S., Thomas A.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Palkovits R.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Palkovits R.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
Pyrene- and Bipyridine-based Covalent Triazine Framework as Versatile Platform for Photocatalytic Solar Fuels Production
CHEMCATCHEM, 2023, p.
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
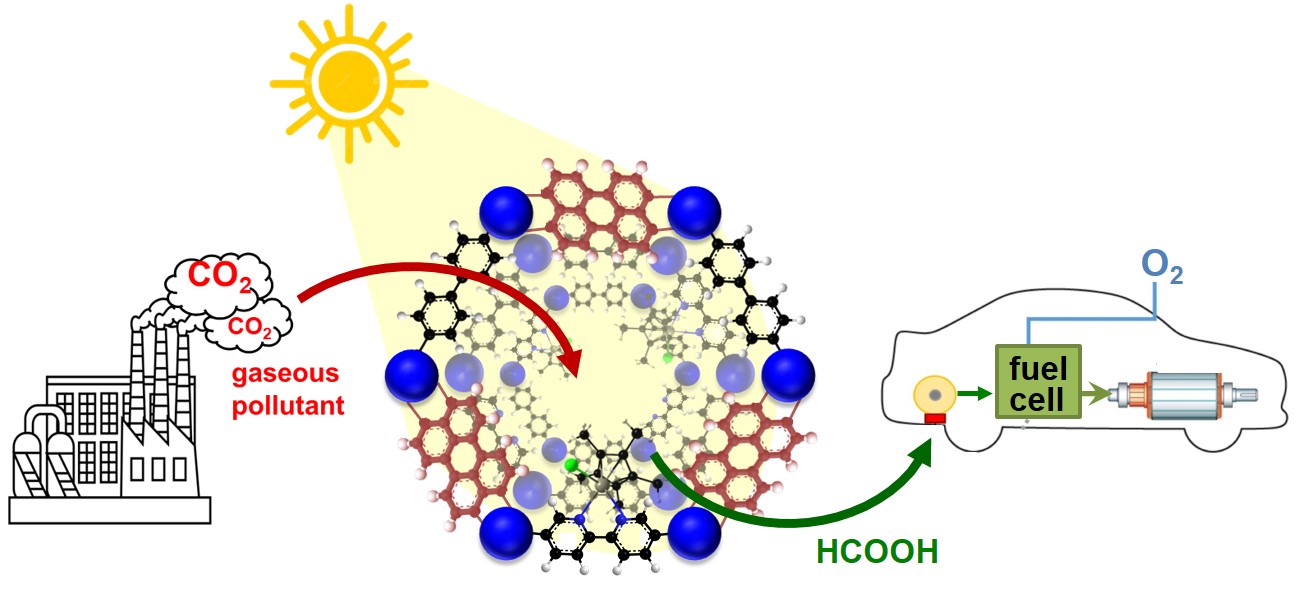
Metal-Organic Framework Catalysts for Solar Fuels: Light-Driven Conversion of Carbon Dioxide into Formic Acid
ACS APPLIED ENERGY MATERIALS, 2023, p.
Perrot V., Roussey A., Benayad A., Veillerot M., Mariolle D., Sole-Daura A., Mellot-Draznieks C., Ricoul F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Jousseaume V.
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Jousseaume V.
ZIF-8 thin films by a vapor-phase process: limits to growth
NANOSCALE, 2023, p.
Samanta P., Sole-Daura A., Rajapaksha R., Wisser F.,  Frederic Meunier Chercheur 445 468 (Standard + 33 [0] 472 445 300) C13.009 Meunier F.,
Frederic Meunier Chercheur 445 468 (Standard + 33 [0] 472 445 300) C13.009 Meunier F.,  Yves Schuurman Chercheur 445 482 (Standard + 33 [0] 472 445 300) P129 Schuurman Y., Sassoye C., Mellot-Draznieks C.,
Yves Schuurman Chercheur 445 482 (Standard + 33 [0] 472 445 300) P129 Schuurman Y., Sassoye C., Mellot-Draznieks C.,  Jerome Canivet Chercheur 445 424 (Standard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Chercheur 445 424 (Standard + 33 [0] 472 445 300) P105 Canivet J.
Heterogenized Molecular Rhodium Phosphine Catalysts within Metal-Organic Frameworks for Alkene Hydroformylation
ACS CATALYSIS, 2023, pp. 4193-4204
Mohr Y., Ranscht A., Alves-Favaro M.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Wisser F.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Wisser F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Nickel-Catalyzed Direct Arylation Polymerization for the Synthesis of Thiophene-Based Cross-linked Polymers
CHEMISTRY-A EUROPEAN JOURNAL, 2022, p.
Henrion M., Mohr Y., Janssens K., Smolders S., Bugaev A., Usoltsev O.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Wisser F., De Vos D.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Wisser F., De Vos D.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Reusable Copper Catechol-based Porous Polymers for the Highly Efficient Heterogeneous Catalytic Oxidation of Secondary Alcohols
CHEMCATCHEM, 2022, p.
Favaro M., Ditz D., Yang J., Bergwinkl S., Ghosh A., Stammler M.,  Chantal Lorentz Technical and administrative staff 445 339 (Switchboard + 33 [0] 472 445 300) P206 Lorentz C., Roeser J.,
Chantal Lorentz Technical and administrative staff 445 339 (Switchboard + 33 [0] 472 445 300) P206 Lorentz C., Roeser J.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Thomas A., Palkovits R.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Thomas A., Palkovits R.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
Finding the Sweet Spot of Photocatalysis-A Case Study Using Bipyridine-Based CTFs
ACS APPLIED MATERIALS & INTERFACES, 2022, 14(12), pp. 14182-14192
Ghosh A., Legrand A., Rajapaksha R., Craig G., Sassoye C., Balazs G.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Furukawa S.,
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Furukawa S.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Wisser F.
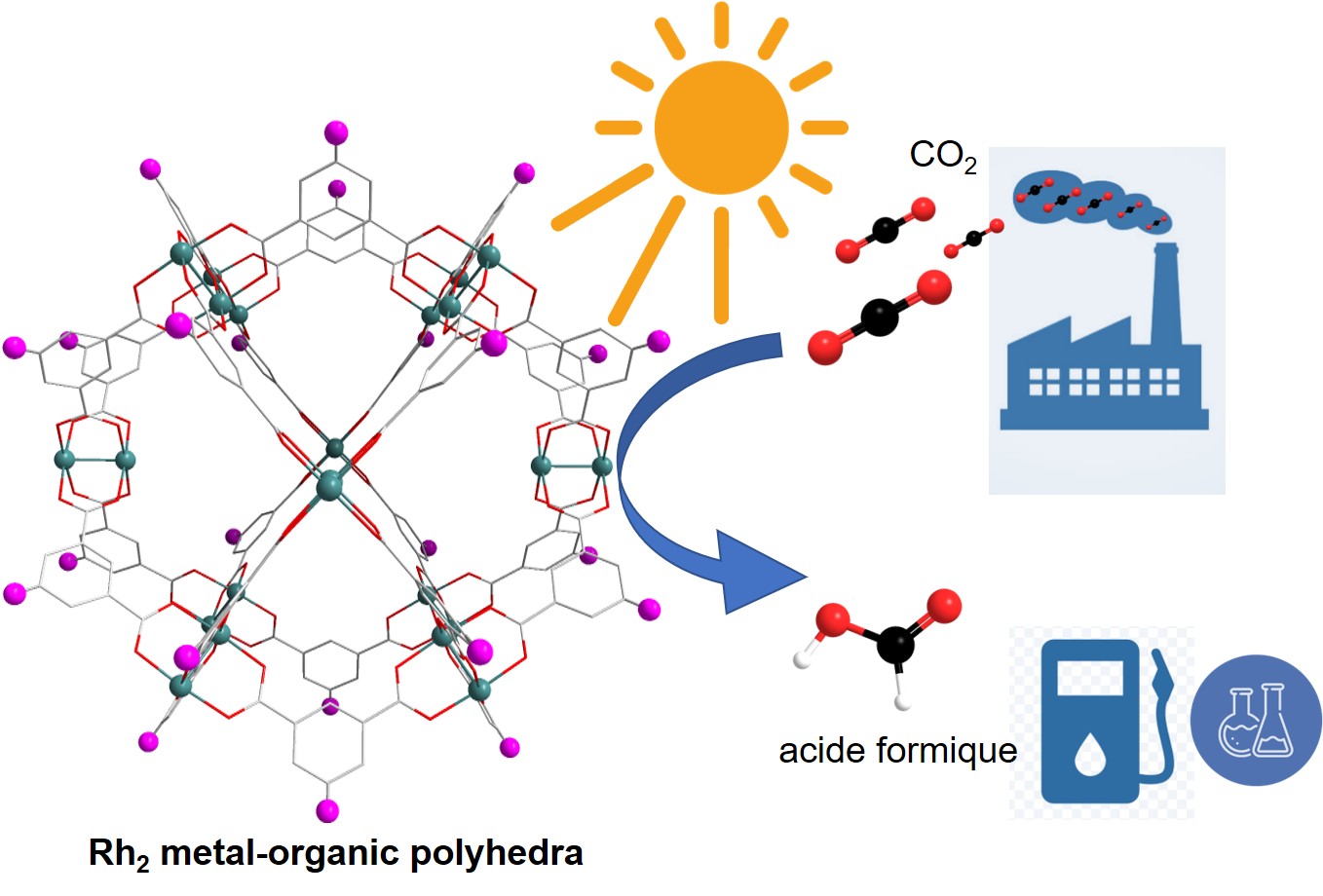
Rhodium-Based Metal-Organic Polyhedra Assemblies for Selective CO2 Photoreduction
JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, 2022, 144(8), pp. 3626-3636
Fonseca A., Heyn R., Froseth M., Thybaut J., Poissonnier J., Meiswinkel A., Zander H.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
A Disruptive Innovation for Upgrading Methane to C3 Commodity Chemicals Technical challenges faced by the C123 European consortium
JOHNSON MATTHEY TECHNOLOGY REVIEW, 2021, 65(2), pp. 311-329
Mohr Y., Alves-Favaro M., Rajapaksha R., Hisler G., Ranscht A., Samanta P.,  Chantal Lorentz Technical and administrative staff 445 339 (Switchboard + 33 [0] 472 445 300) P206 Lorentz C., Duguet M., Mellot-Draznieks C.,
Chantal Lorentz Technical and administrative staff 445 339 (Switchboard + 33 [0] 472 445 300) P206 Lorentz C., Duguet M., Mellot-Draznieks C.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Wisser F.,
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Wisser F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
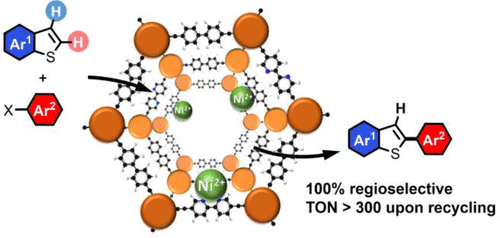
Heterogenization of a Molecular Ni Catalyst within a Porous Macroligand for the Direct C-H Arylation of Heteroarenes
ACS CATALYSIS, 2021, 11(6), pp. 3507-3515
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bernoud E., Bonnefoy J., Legrand A., Todorova T.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bernoud E., Bonnefoy J., Legrand A., Todorova T.,  Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Mellot-Draznieks C.
Elsje Alessandra Quadrelli Researcher 445 359 (Switchboard + 33 [0] 472 445 300) P114 Quadrelli E. A., Mellot-Draznieks C.
Synthetic and computational assessment of a chiral metal-organic framework catalyst for predictive asymmetric transformation
Chemical science, 2020, 11, pp. 8800-8808
Wisser F., Duguet M., Perrinet Q., Ghosh A., Alves-Favaro M., Mohr Y.,  Chantal Lorentz Technical and administrative staff 445 339 (Switchboard + 33 [0] 472 445 300) P206 Lorentz C., Quadrelli E., Palkovits R.,
Chantal Lorentz Technical and administrative staff 445 339 (Switchboard + 33 [0] 472 445 300) P206 Lorentz C., Quadrelli E., Palkovits R.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Mellot-Draznieks C., De Waele V.,
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Mellot-Draznieks C., De Waele V.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Molecular Porous Photosystems Tailored for Long-Term Photocatalytic CO2 Reduction
Angewandte chemie-international edition, 2020, 59, pp. 5116-5122
Mohr Y., Hisler G., Grousset L., Roux Y., Quadrelli E., Wisser F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Nickel-catalyzed and Li-mediated regiospecific C-H arylation of benzothiophenes
Green chemistry, 2020, 22, pp. 3155-3161
Mohr Y., Renom-Carrasco M., Demarcy C., Quadrelli E., Camp C., Wisser F., Clot E., Thieuleux C.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Regiospecificity in Ligand-Free Pd-Catalyzed C-H Arylation of Indoles: LiHMDS as Base and Transient Directing Group
Acs catalysis, 2020, 10, pp. 2713-2719
Wisser F., Mohr Y., Quadrelli E.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
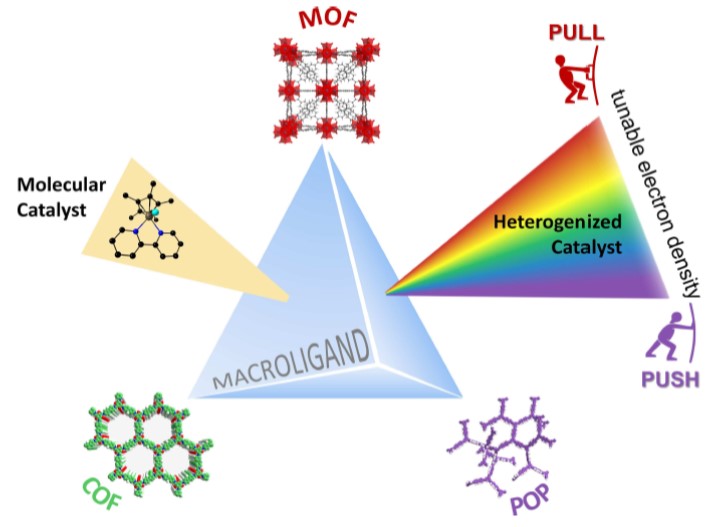
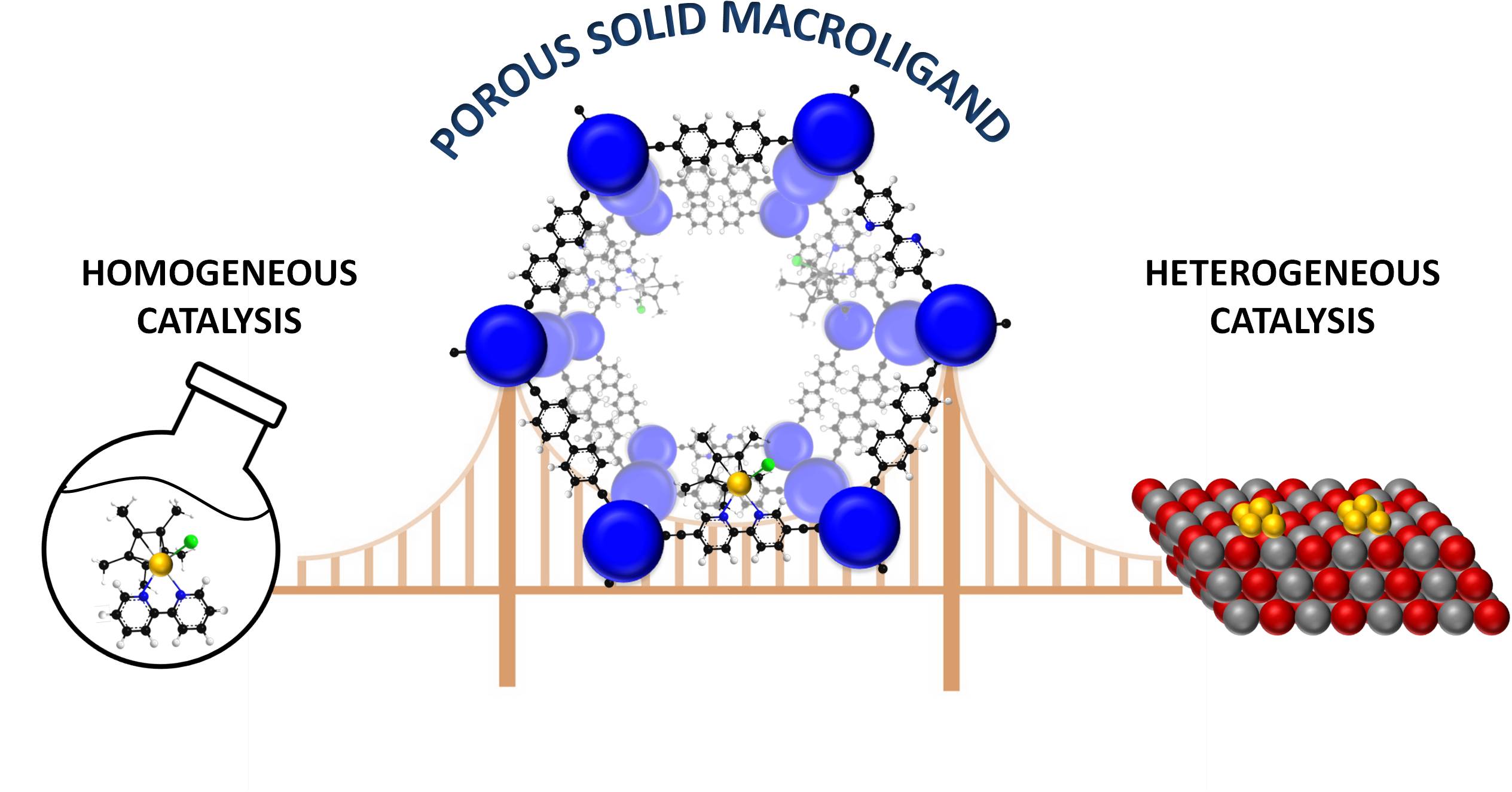
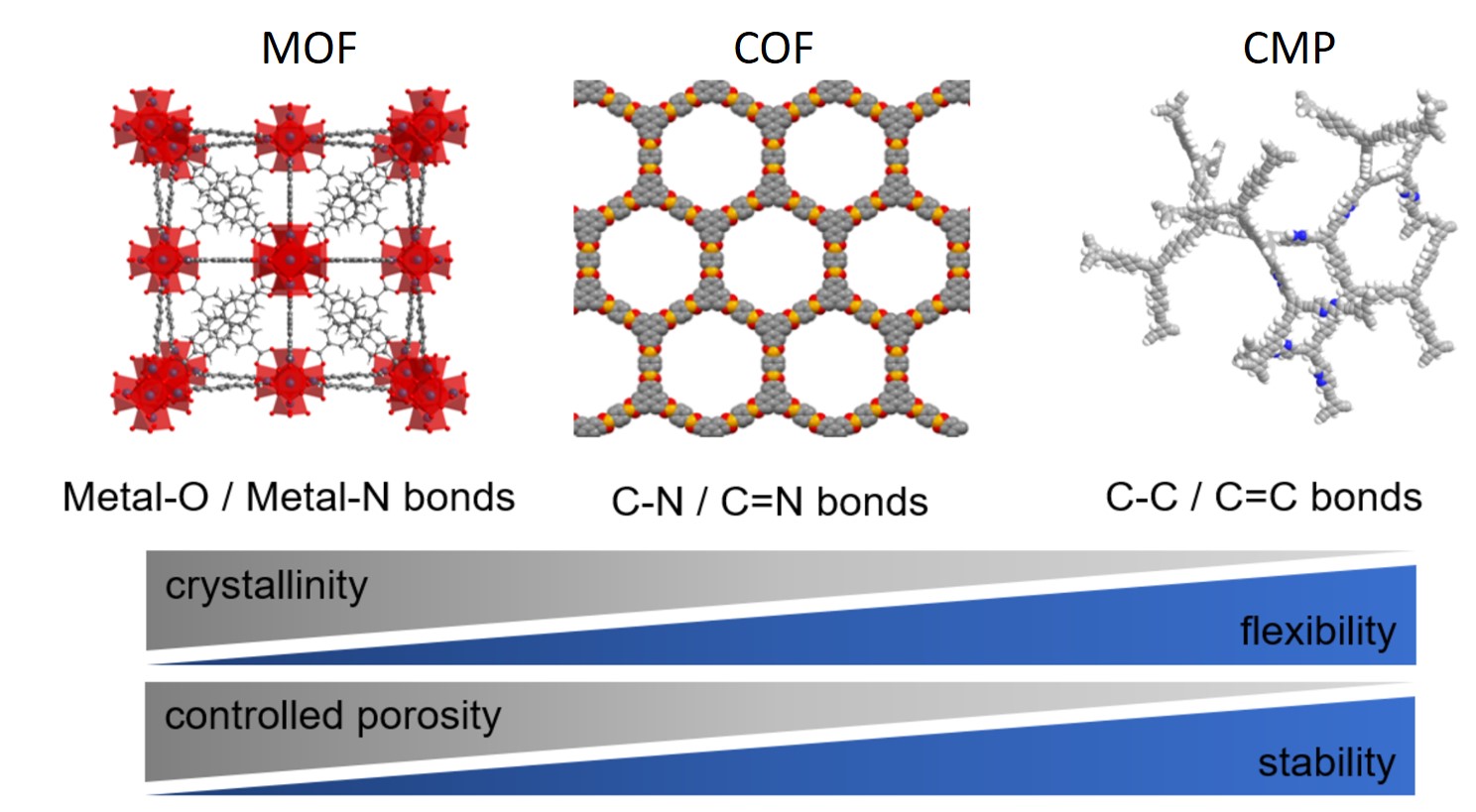
Porous Macroligands: Materials for Heterogeneous Molecular Catalysis
Chemcatchem, 2020, 5, pp. 1270-1275
Wang X., Wisser F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Fontecave M., Mellot-Draznieks C.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Fontecave M., Mellot-Draznieks C.
Immobilization of a Full Photosystem in the Large-Pore MIL-101 Metal-Organic Framework for CO2 reduction
Chemsuschem, 2018, 11, pp. 3315-3322
Wisser F., Mohr Y., Quadrelli E.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.,
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Microporous Polymers as Macroligands for Pentamethylcyclopentadienylrhodium Transfer-Hydrogenation Catalysts
Chemcatchem, 2018, 10, pp. 1778-1782
Wisser F., Berruyer P.,  Luis Cardenas Researcher 445 314 (Switchboard + 33 [0] 472 445 300) P205 Cardenas L., Mohr Y., Quadrelli E., Lesage A.,
Luis Cardenas Researcher 445 314 (Switchboard + 33 [0] 472 445 300) P205 Cardenas L., Mohr Y., Quadrelli E., Lesage A.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.,
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.
Hammett Parameter in Microporous Solids as Macroligands for Heterogenized Photocatalysts
Acs catalysis, 2018, 8, pp. 1653-1661
Robin J., Audebrand N., Poriel C.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Calvez G., Roisnel T., Dorcet V., Roussel P.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Calvez G., Roisnel T., Dorcet V., Roussel P.
A series of chiral metal-organic frameworks based on fluorene di- and tetra-carboxylates: syntheses, crystal structures and luminescence properties
Crystengcomm, 2017, 19, pp. 2042-2056
Marepally B, Ampelli C, Genovese C, Saboo T, Perathoner S, Wisser F, Veyre L,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Quadrelli E, Centi G
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Quadrelli E, Centi G
Enhanced formation of > C1 Products in Electroreduction of CO2 by Adding a CO2 Adsorption Component to a Gas-Diffusion Layer-Type Catalytic Electrode
Chemsuschem, 2017, 10, pp. 4442-4446
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Lysenko V, Lehtinen J, Legrand A, Wisser F, Quadrelli E,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Lysenko V, Lehtinen J, Legrand A, Wisser F, Quadrelli E,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Sensitive Photoacoustic IR Spectroscopy for the Characterization of Amino/Azido Mixed-Linker Metal-Organic Frameworks
Chemphyschem, 2017, 18, pp. 2855-2858
Dhainaut J., Avci-Camur C., Troyano J., Legrand A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Imaz I., Maspoch D., Reinsch H.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Imaz I., Maspoch D., Reinsch H.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Systematic study of the impact of MOF densification into tablets on textural and mechanical properties
CrystEngComm, 2017, 19, pp. 4211-4218
Todorova T. K., Rozanska X., Gervais C., Legrand A., Ho L. N., Berruyer P., Lesage A., Emsley L.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.,
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Mellot-Draznieks C.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Mellot-Draznieks C.
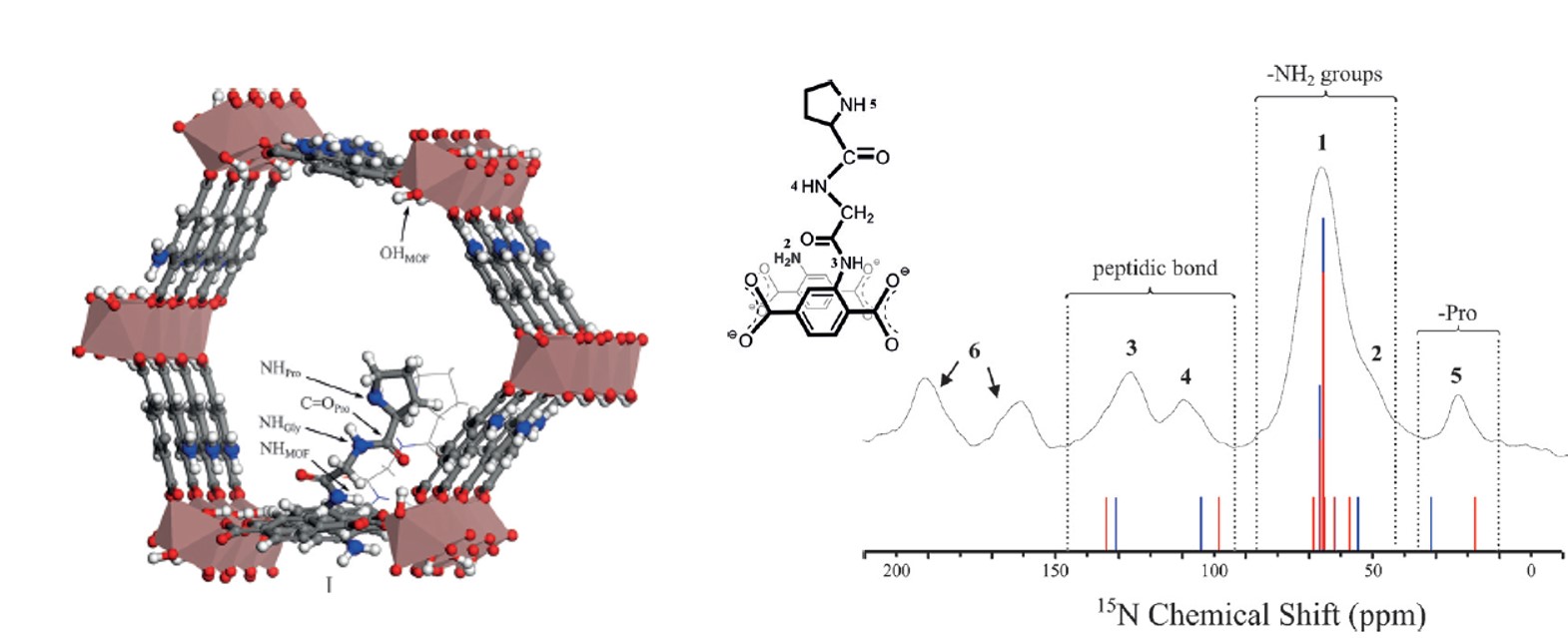
Molecular Level Characterization of the Structure and Interactions in Peptide-Functionalized Metal-Organic Frameworks
Chemistry - A European Journal, 2016, 22, pp. 16531-16538
Legrand A., Pastushenko A., Lysenko V., Geloen A., Quadrelli E. A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Enhanced Ligand-Based Luminescence in Metal-Organic Framework Sensor
Chemnanomat, 2016, 2, pp. 866-872
Aguado S., El-Jamal S.,  Frederic Meunier Researcher 445 468 (Switchboard + 33 [0] 472 445 300) C13.009 Meunier F.,
Frederic Meunier Researcher 445 468 (Switchboard + 33 [0] 472 445 300) C13.009 Meunier F.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
A Pt/Al2O3-supported metal-organic framework film as the size-selective core-shell hydrogenation catalyst
Chemical Communications, 2016, 52, pp. 7161-7163
Hendon Christopher H., Bonnefoy J., Quadrelli Elsje Alessandra,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Chambers Matthew B., Rousse G., Walsh A., Fontecave M., Mellot-Draznieks C.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Chambers Matthew B., Rousse G., Walsh A., Fontecave M., Mellot-Draznieks C.
A Simple and Non-Destructive Method for Assessing the Incorporation of Bipyridine Dicarboxylates as Linkers within Metal-Organic Frameworks
Chemistry - A European Journal, 2016, p.
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Vandichel M.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Vandichel M.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Origin of highly active metal-organic framework catalysts: defects? Defects!
Dalton transactions (Cambridge, England : 2003), 2016, 45, pp. 4090-9
Bonnefoy J., Legrand A., Quadrelli E. A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Enantiopure Peptide-Functionalized Metal-Organic Frameworks
Journal of the American Chemical Society, 2015, 137, pp. 9409-9416
Baias M., Lesage A., Aguado S.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Moizan-Baslé V., Audebrand N.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Moizan-Baslé V., Audebrand N.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Emsley L.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Emsley L.
Superstructure of a Substituted Zeolitic Imidazolate Metal–Organic Framework Determined by Combining Proton Solid-State NMR Spectroscopy and DFT Calculations
Angewandte Chemie International Edition, 2015, 54, pp. 5971--5976
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Proline-functionalized metal-organic frameworks and their use in asymmetric catalysis: pitfalls in the MOFs rush
RSC Advances, 2015, 5, pp. 11254-11256
Chambers M. B., Wang X., Elgrishi N., Hendon C. H., Walsh A., Bonnefoy J.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Quadrelli E. A.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Quadrelli E. A.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Mellot-Draznieks C., Fontecave M.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D., Mellot-Draznieks C., Fontecave M.
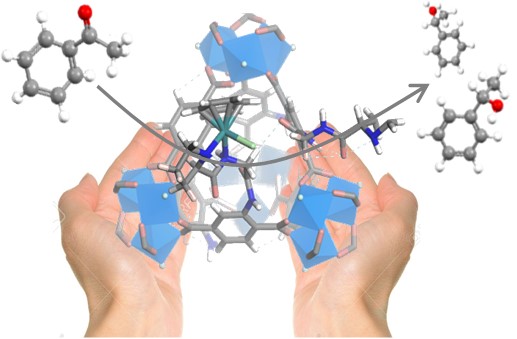
Photocatalytic Carbon Dioxide Reduction with Rhodium-based Catalysts in Solution and Heterogenized within Metal-Organic Frameworks
Chemsuschem, 2015, 8, pp. 603-608
Savonnet M., Camarata A.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bazer-Bachi D., Bats N., Lecocq V.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Bazer-Bachi D., Bats N., Lecocq V.,  Catherine Pinel Researcher 445 478 (Switchboard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Researcher 445 478 (Switchboard + 33 [0] 472 445 300) P201 Pinel C.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Tailoring metal-organic framework catalysts by click chemistry
Dalton Transactions, 2012, 41, pp. 3945-3948
Yamamoto T., Muto K., Komiyama M.,  Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Yamaguchi J., Itami K.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Yamaguchi J., Itami K.
Nickel-Catalyzed C-H Arylation of Azoles with Haloarenes: Scope, Mechanism, and Applications to the Synthesis of Bioactive Molecules
Chemistry A - European Journal, 2011, 17, pp. 10113-10122
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Aguado S., Bergeret G.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Aguado S., Bergeret G.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Amino acid functionalized metal-organic frameworks by a soft coupling-deprotection sequence
Chemical Communications, 2011, 47, pp. 11650-11652
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Aguado S.,
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Aguado S.,  Cecile Daniel Technical and administrative staff 445 365 (Switchboard + 33 [0] 472 445 300) P109 Daniel C.,
Cecile Daniel Technical and administrative staff 445 365 (Switchboard + 33 [0] 472 445 300) P109 Daniel C.,  David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
David Farrusseng Researcher 445 365 (Switchboard + 33 [0] 472 445 300) P109 Farrusseng D.
Engineering the Environment of a Catalytic Metal-Organic Framework by Postsynthetic Hydrophobization
ChemCatChem, 2011, 3, pp. 675-678
 Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Yamaguchi J., Ban I., Itami, K.
Jerome Canivet Researcher 445 424 (Switchboard + 33 [0] 472 445 300) P105 Canivet J., Yamaguchi J., Ban I., Itami, K.
Nickel-catalyzed biaryl coupling of heteroarenes and aryl halides/triflates
Organic Letters, 2009, 11, pp. 1733-1736