Fouad Elgayar Personnel étudiant ou non permanent 445 300 (Standard + 33 [0] 472 445 300) P-1406 Elgayar F., Jeanneau E., Bargiela P.,
Fouad Elgayar Personnel étudiant ou non permanent 445 300 (Standard + 33 [0] 472 445 300) P-1406 Elgayar F., Jeanneau E., Bargiela P.,  Adel Mesbah Chercheur 445 489 (Standard + 33 [0] 472 445 300) P-1308 Mesbah A.,
Adel Mesbah Chercheur 445 489 (Standard + 33 [0] 472 445 300) P-1308 Mesbah A.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Exploring untapped potential of the molecular precursor approach to control fluorine-doping in mesostructured titania for acid catalysis
CATALYSIS SCIENCE & TECHNOLOGY, 2025, p.
Purohit B., Galal S., Jeanneau E., Guyot Y., Amans D., Mahler B., Dujardin C., Ledoux G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Remarkably stable luminescence thermometric performance of upconverting LiYF4:Yb3+, Tm3+ nanoparticles obtained via an anhydrous precursor strategy
POLYHEDRON, 2025, 277, p.
Bhattacharyya B., Kashyap S., Begum T., Borah G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Islam N., Gogoi N.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Islam N., Gogoi N.
A convenient bottom up route to covalently anchor Pd(II)-PEPPSI on layered zirconium(IV) phosphonate: Efficient catalyst for Suzuki-Miyaura cross coupling of aryl chlorides
MOLECULAR CATALYSIS, 2024, 569, p.
Salem R.,  Marion Eternot Personnel technique et administratif 445 375 (Standard + 33 [0] 472 445 300) P1303 Eternot M., Purohit B., Bargiela P.,
Marion Eternot Personnel technique et administratif 445 375 (Standard + 33 [0] 472 445 300) P1303 Eternot M., Purohit B., Bargiela P.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Innovative Synthesis of YF3-TiO2 Acid-Base Catalysts with High Surface Area by Embedding Yttrium Trifluoride Nanoparticles in TiO2 Metallogel
CHEMCATCHEM, 2024, p.
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Gahlot S., Raydan N., Burel L.,
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Gahlot S., Raydan N., Burel L.,  Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T., Bonhomme A., Bargiela P.
Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T., Bonhomme A., Bargiela P.
Single Source Precursor Path to 2D Materials: A Case Study of Solution-Processed Molybdenum-Rich MoSe2-x Ultrathin Nanosheets
CHEMNANOMAT, 2024, p.
Soussi K., Jeanneau E., Maldivi P., Clemancey M., Latour J., Khrouz L.,  Chantal Lorentz Personnel technique et administratif 445 339 (Standard + 33 [0] 472 445 300) P206 Lorentz C., Daniele S.,
Chantal Lorentz Personnel technique et administratif 445 339 (Standard + 33 [0] 472 445 300) P206 Lorentz C., Daniele S.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
N-Alkyl substituted triazenide-bridged homoleptic iron(II) dimers with an exceptionally short Fe-Fe bond
DALTON TRANSACTIONS, 2023, 53 (4), pp. 1439-1444
Neto Antonio De Brito S., Oliveira A.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.
Sol-Gel controlled dispersion of Nb within TiO2 to obtain water tolerant catalysts with tunable acid-base properties for dihydroxyacetone transformation
APPLIED CATALYSIS A-GENERAL, 2023, 658, p.
Ben Salem R.,  Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T., Bargiela P., Lebeau B.,
Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T., Bargiela P., Lebeau B.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.
Fluorinated Zirconia Prepared by Anionic Exchange of Zirconium Oxo/Hydroxide with Trifluoroacetic Acid: Structure, Acid-Base and Catalytic Properties
CHEMCATCHEM, 2023, p.
Cottrino S., Gaudisson T., Pailhes S., Ferrara E.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Mezouar M., Largeteau A., Le Godec Y., Le Floch S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Mezouar M., Largeteau A., Le Godec Y., Le Floch S.
In situ X-ray diffraction study of a TiO2 nanopowder Spark Plasma Sintering under very high pressure
JOURNAL OF THE EUROPEAN CERAMIC SOCIETY, 2023, 43(7), pp. 2783-2793
Purohit B., Jeanneau E., Guyot Y., Amans D., Mahler B., Joubert M., Dujardin C., Ledoux G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
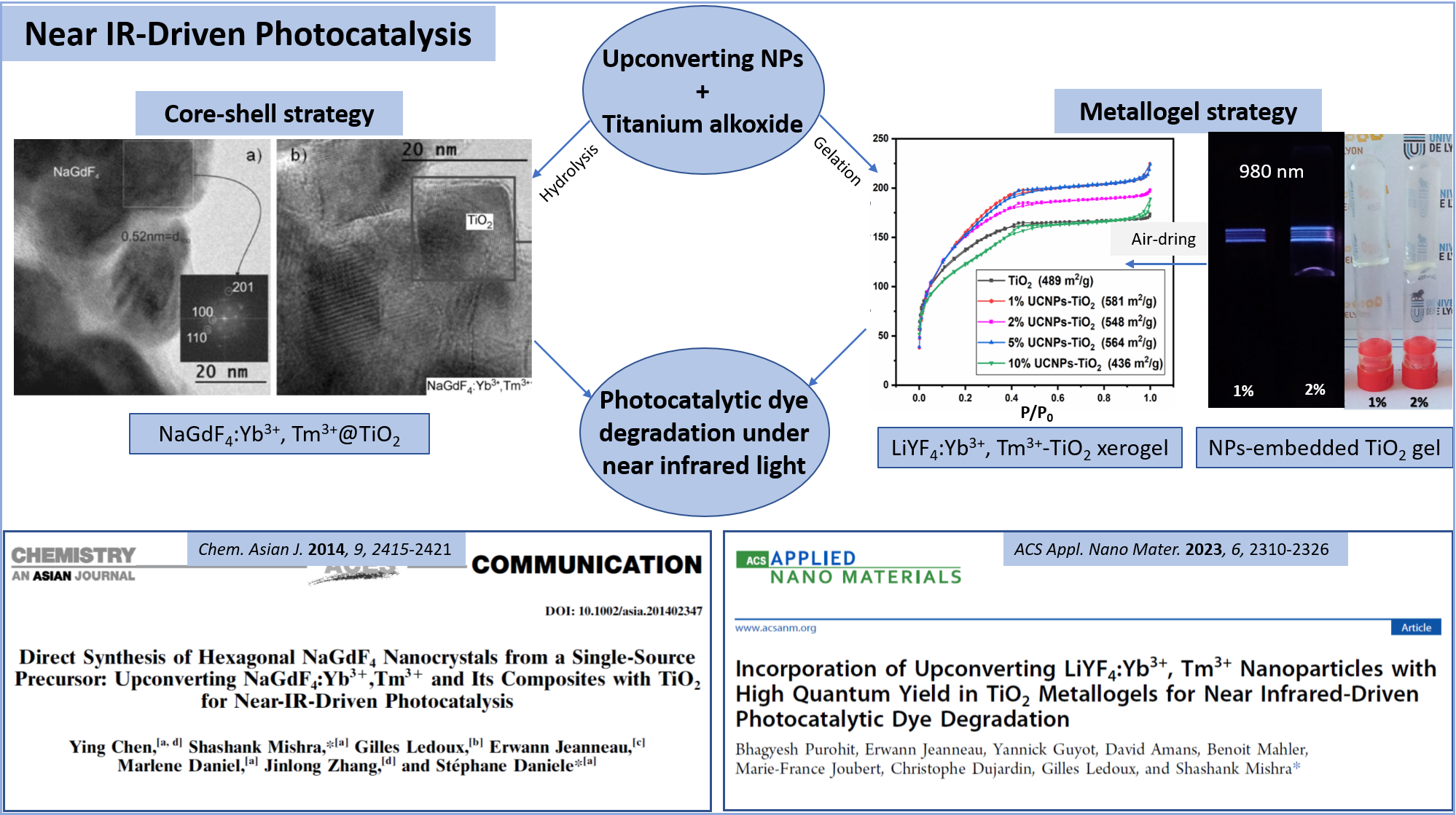
Incorporation of Upconverting LiYF4:Yb3+, Tm3+ Nanoparticles with High Quantum Yield in TiO2 Metallogels for Near Infrared-Driven Photocatalytic Dye Degradation
ACS APPLIED NANO MATERIALS, 2023, 6(4), pp. 2310-2326
Purohit B., Jeanneau E.,  Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T., Ledoux G.,
Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T., Ledoux G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
An anhydrous precursor approach to BaYF5-based upconverting nanocrystals
JOURNAL OF THE INDIAN CHEMICAL SOCIETY, 2022, 99(2), p. 100322
Machon D., Le Floch S.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Masenelli-Varlot K., Hermet P., Melinon P.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Masenelli-Varlot K., Hermet P., Melinon P.
Extreme structural stability of Ti0.5Sn0.5O2 nanoparticles: synergistic effect in the cationic sublattice
NANOSCALE, 2022, 14(38), pp. 14286-14296
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Ultra-mild synthesis of nanometric metal chalcogenides using organyl chalcogenide precursors
CHEMICAL COMMUNICATIONS, 2022, p.
Kumar D., Chouhan A., Jeanneau E.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Pandey A.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Pandey A.
Syntheses and characterizations of calcium and strontium based coordination compounds with the 5-(2-pyridyl)tetrazolate ligand, respectively exhibiting extended 1 D and 2 D structures
JOURNAL OF MOLECULAR STRUCTURE, 2022, 1260, p.
Bhattacharyya B.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Gogoi N.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Gogoi N.
Accessing cationic zirconium phosphonate nanosheets for anion exchange applications
INORGANICA CHIMICA ACTA, 2022, 531, p.
Brune V., Grosch M., Weissing R., Hartl F., Frank M.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Mathur S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Mathur S.
Influence of the choice of precursors on the synthesis of two-dimensional transition metal dichalcogenides
DALTON TRANSACTIONS, 2021, 50(36), pp. 12365-12385
Brune V., Raydan N., Sutorius A., Hartl F., Purohit B., Gahlot S., Bargiela P., Burel L., Wilhelm M., Hegemann C., Atamturk U., Mathur S.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Single source precursor route to nanometric tin chalcogenides
DALTON TRANSACTIONS, 2021, p.
Atamturk U., Brune V.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Mathur S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Mathur S.
Vapor Phase Synthesis of SnS Facilitated by Ligand-Driven "Launch Vehicle" Effect in Tin Precursors
MOLECULES, 2021, 26(17), p.
Gahlot S.,  Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,
Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,  Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S.,
Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S.,  Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C.
Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C.
High surface area g-C3N4 and g-C3N4-TiO2 photocatalytic activity under UV and Visible light: Impact of individual component
JOURNAL OF ENVIRONMENTAL CHEMICAL ENGINEERING, 2021, 9(4), p.
Gahlot S., Purohit B., Jeanneau E.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
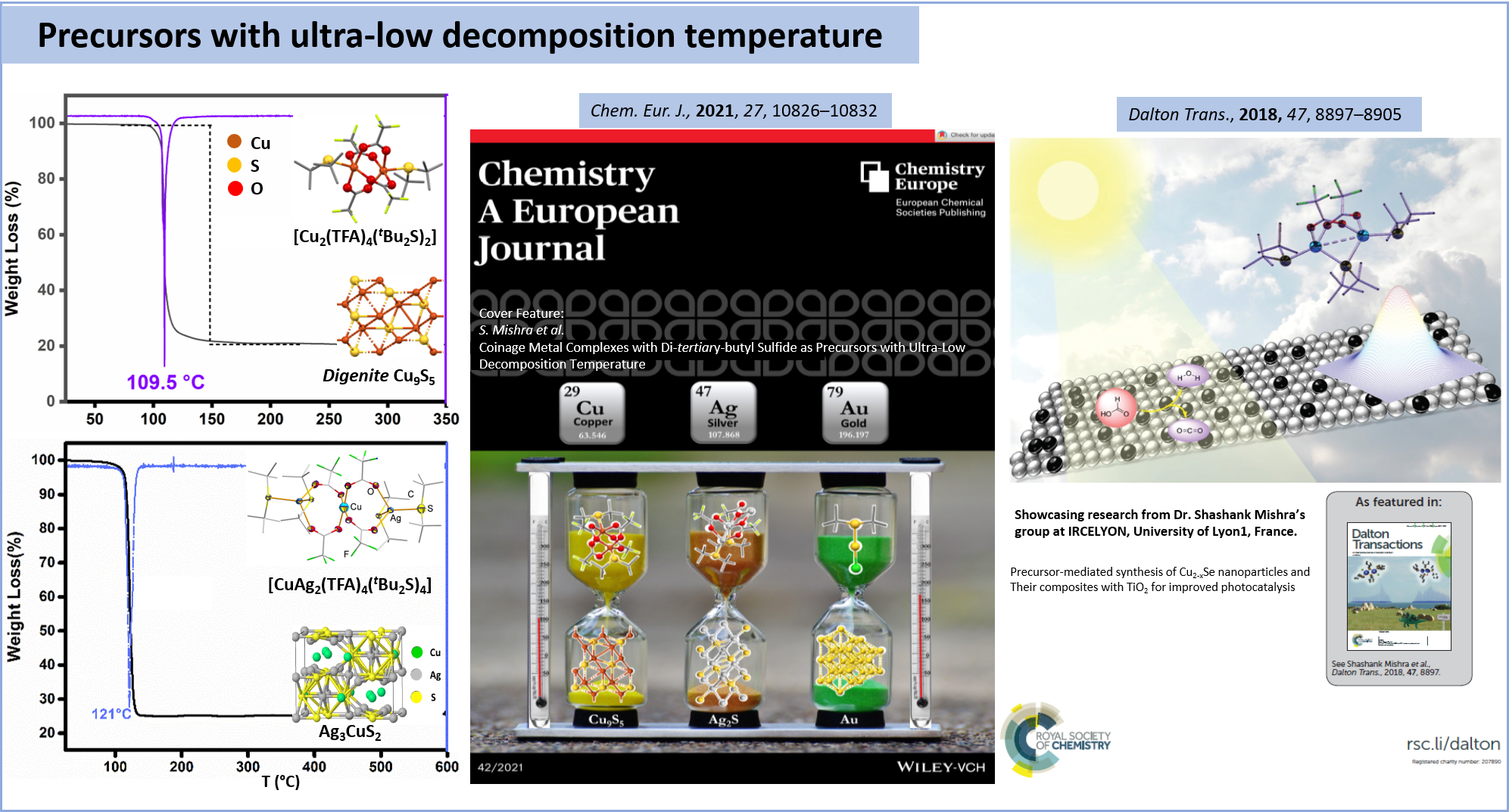
Coinage Metal Complexes with Di-tertiary-butyl Sulfide as Precursors with Ultra-Low Decomposition Temperature
CHEMISTRY-A EUROPEAN JOURNAL, 2021, 27(42), pp. 10826-10832
Bijou D.,  Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T.,
Thibaut Cornier Personnel technique et administratif 445 335 (Standard + 33 [0] 472 445 300) P1414 Cornier T.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Merzoud L., Chermette H., Jeanneau E., Maudez W., Benvenuti G., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Merzoud L., Chermette H., Jeanneau E., Maudez W., Benvenuti G., Daniele S.
Synthesis and Thermal Behavior of Heteroleptic gamma-Substituted Acetylacetonate-Alkoxides of Titanium
EUROPEAN JOURNAL OF INORGANIC CHEMISTRY, 2021, 2021(20), pp. 1976-1983
Neto A., Alves Da Cruz M., Jeanneau E., Oliveira A.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
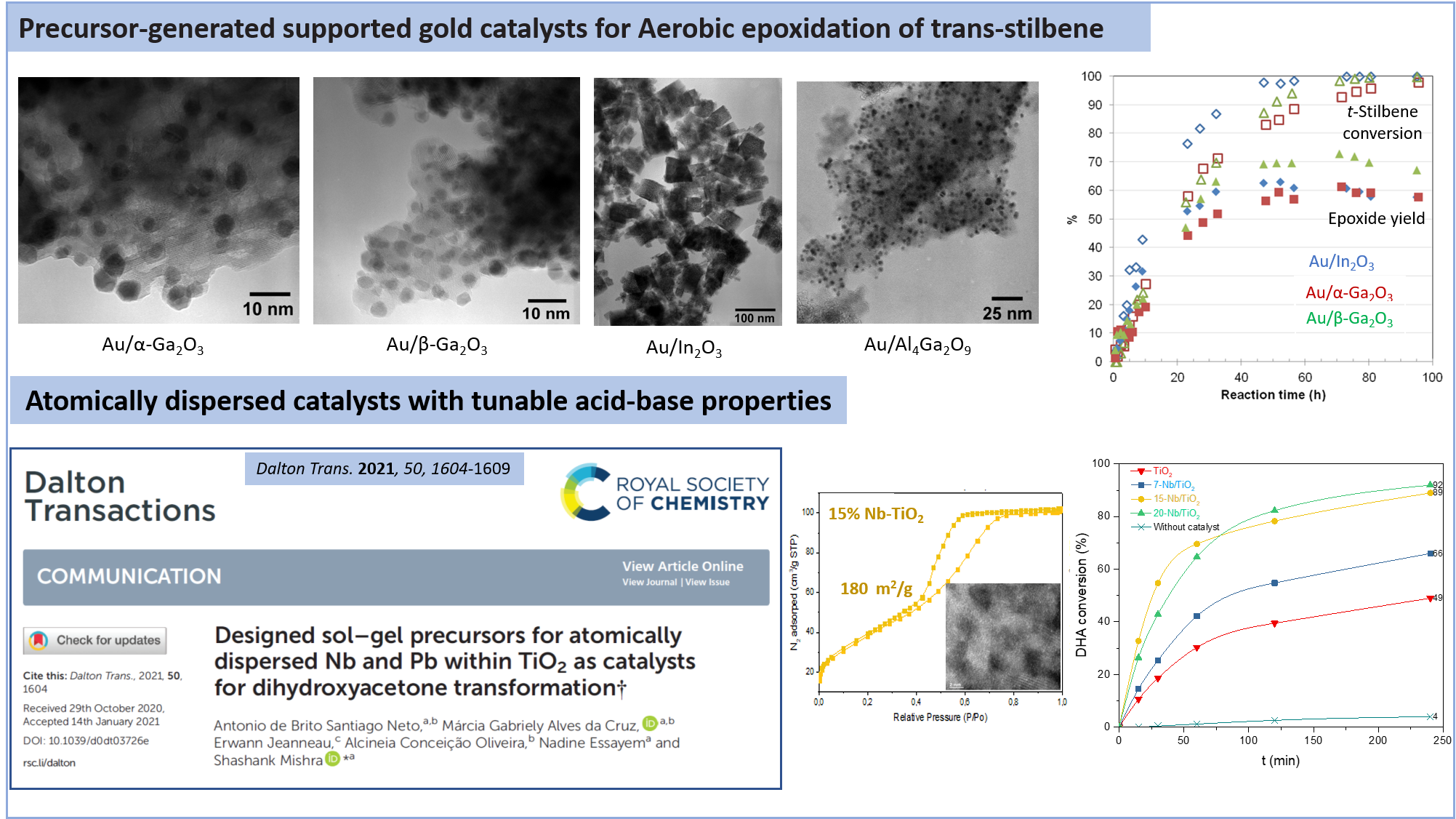
Designed sol-gel precursors for atomically dispersed Nb and Pb within TiO2 as catalysts for dihydroxyacetone transformation
DALTON TRANSACTIONS, 2021, 50(5), pp. 1604-1609
Verchère A., Cottrino S., Fantozzi G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Gaudisson T., Blanchard N., Pailhes S., Daniele S., Le Floch S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Gaudisson T., Blanchard N., Pailhes S., Daniele S., Le Floch S.
Effect of High Pressure Spark Plasma Sintering on the Densification of a Nb-Doped TiO2 Nanopowder
Ceramics, 2020, 3(4), pp. 507-520
Purohit B., Amans D., Guyot Y., Mahler B., Joubert M., Dujardin C., Daniele S., Ledoux G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
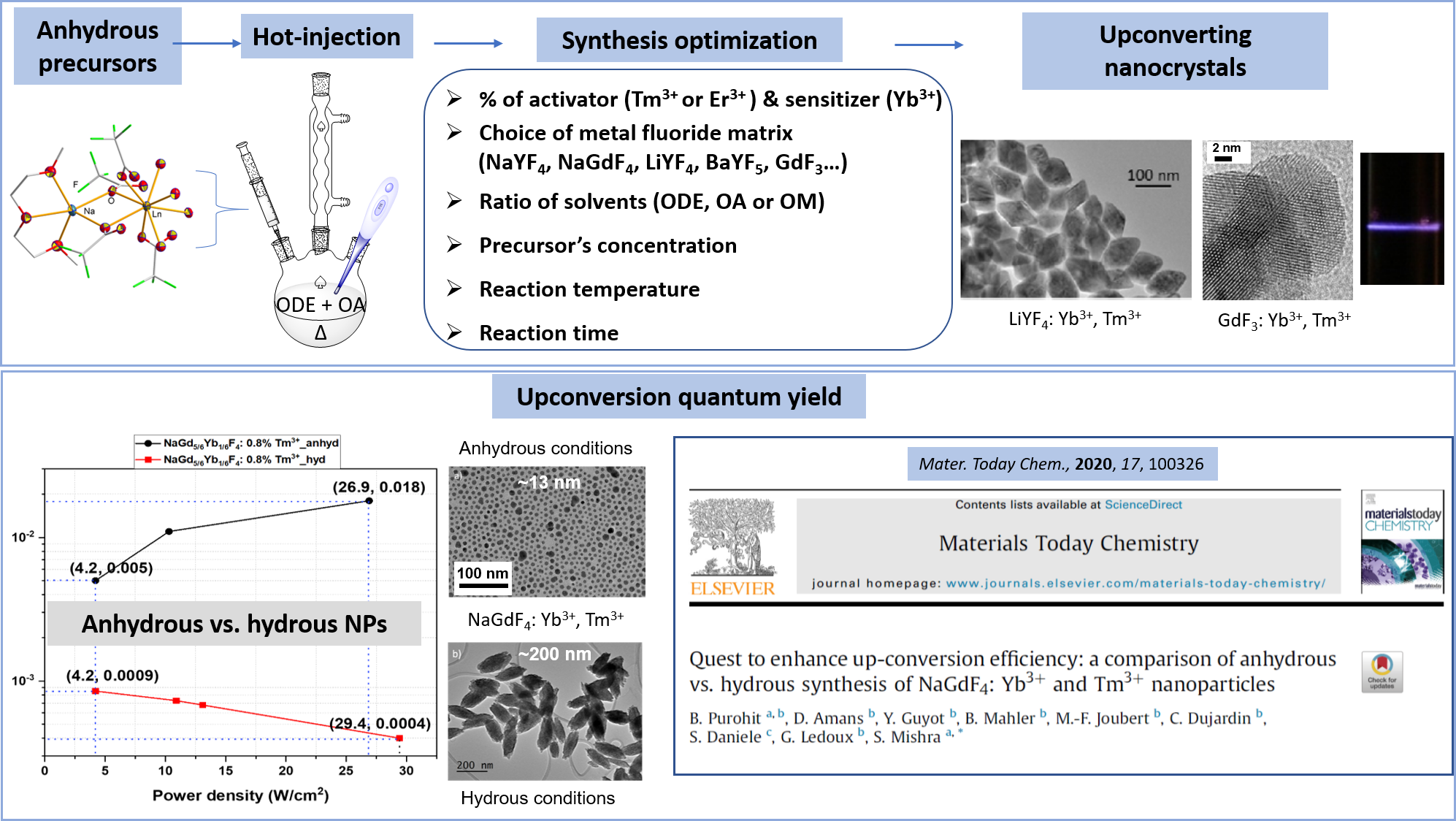
Quest to enhance up-conversion efficiency: a comparison of anhydrous vs. hydrous synthesis of NaGdF4: Yb3+ and Tm3+ nanoparticles
Materials today chemistry, 2020, 17, p. 100326
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S.
Molecular Engineering of Metal Alkoxides for Solution Phase Synthesis of High-Tech Metal Oxide Nanomaterials
Chemistry-a european journal, 2020, 42 , pp. 9292-9303
Verchère A., Pailhes S., Le Floch S., Cottrino S., Debord R., Fantozzi G., Misra S., Candolfi C., Lenoir B., Daniele S.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
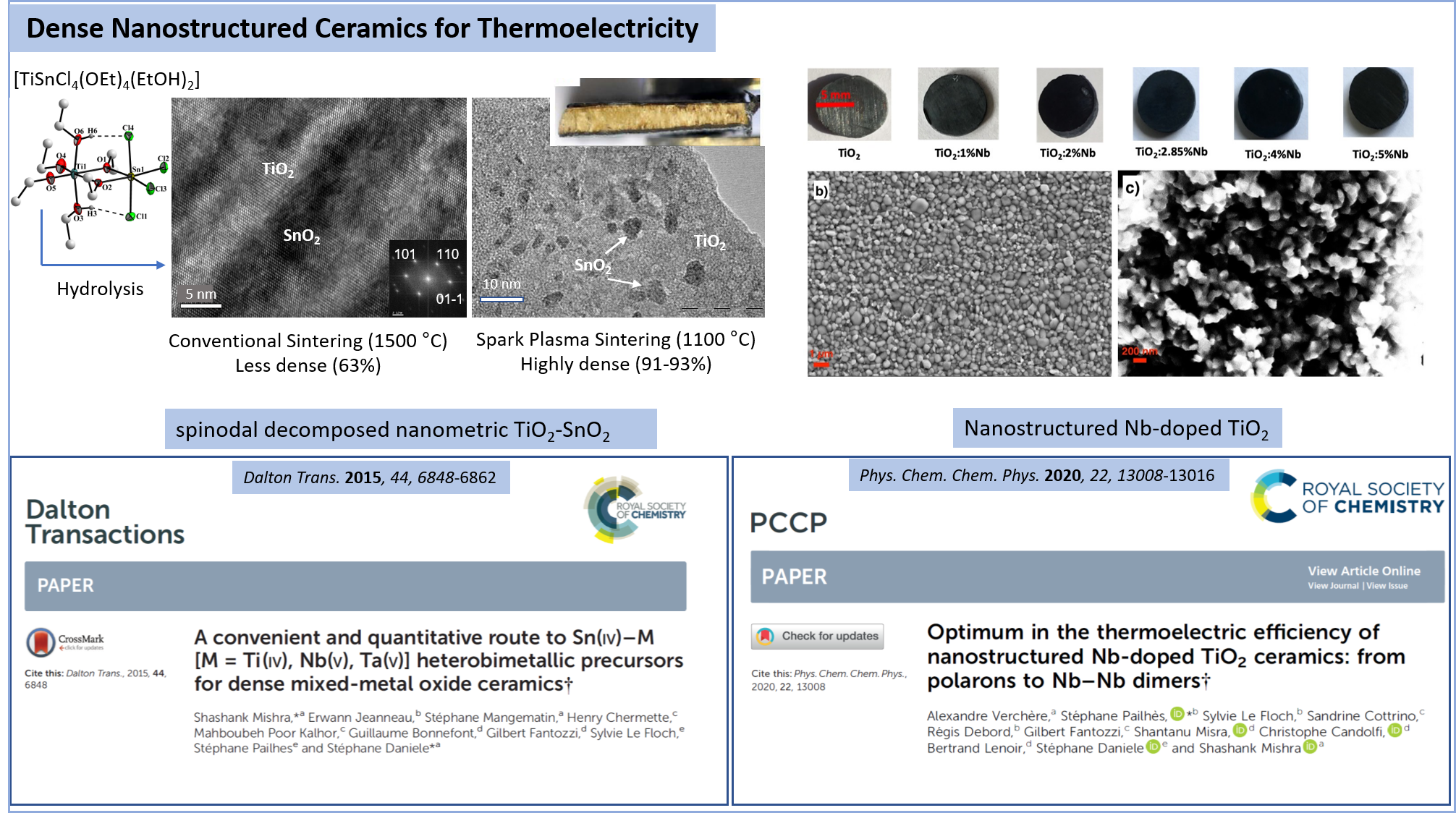
Optimum in the thermoelectric efficiency of nanostructured Nb-doped TiO 2 ceramics: from polarons to Nb–Nb dimers
Physical chemistry chemical physics, 2020, 22, pp. 13008-13016
Gahlot S., Jeanneau E., Singh D., Panda P., Mishra Y., Ahuja R., Ledoux G.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
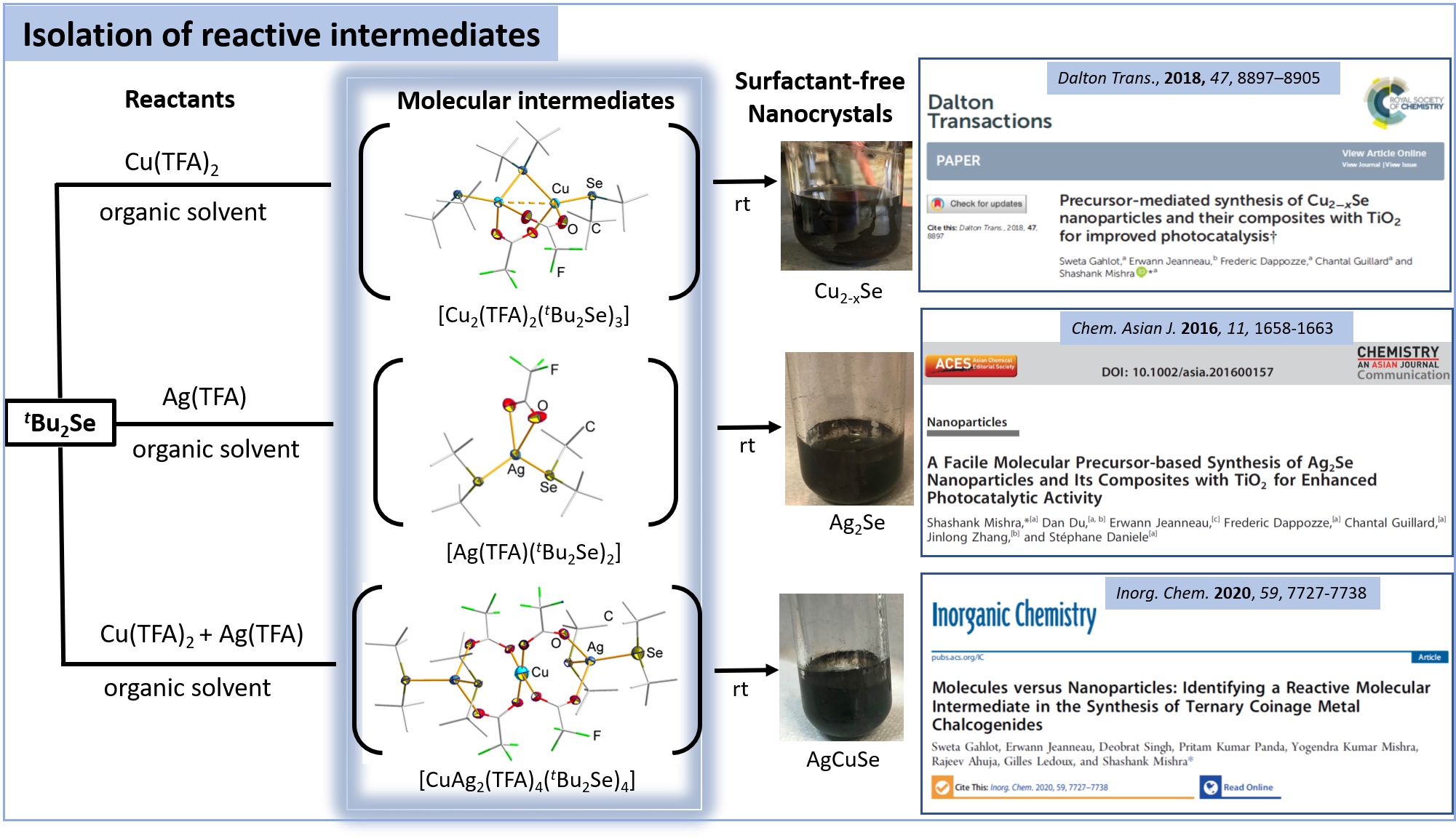
Molecules versus Nanoparticles: Identifying a Reactive Molecular Intermediate in the Synthesis of Ternary Coinage Metal Chalcogenides
Inorganic chemistry, 2020, 59, pp. 7727-7738
Verchere A.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Guillon H., Decams J., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Guillon H., Decams J., Daniele S.
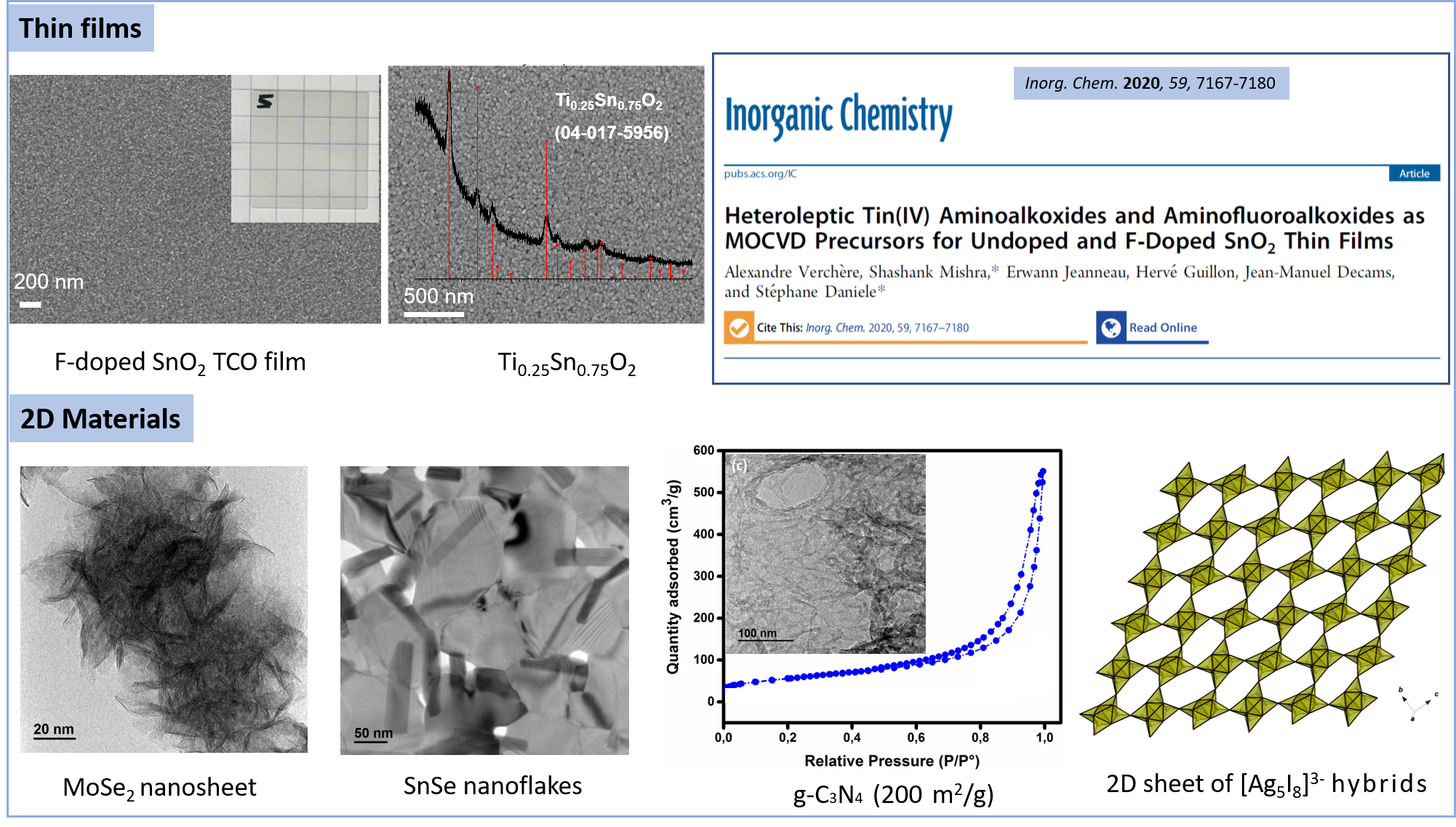
Heteroleptic Tin(IV) Aminoalkoxides and Aminofluoroalkoxides as MOCVD Precursors for Undoped and F-Doped SnO2 Thin Films
Inorganic chemistry, 2020, 59, pp. 7167-7180
Gahlot S.,  Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F., Singh D., Ahuja R.,
Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F., Singh D., Ahuja R.,  Luis Cardenas Researcher 445 314 (Switchboard + 33 [0] 472 445 300) P205 Cardenas L., Burel L., Amans D.,
Luis Cardenas Researcher 445 314 (Switchboard + 33 [0] 472 445 300) P205 Cardenas L., Burel L., Amans D.,  Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C.,
Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C.,  Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S.
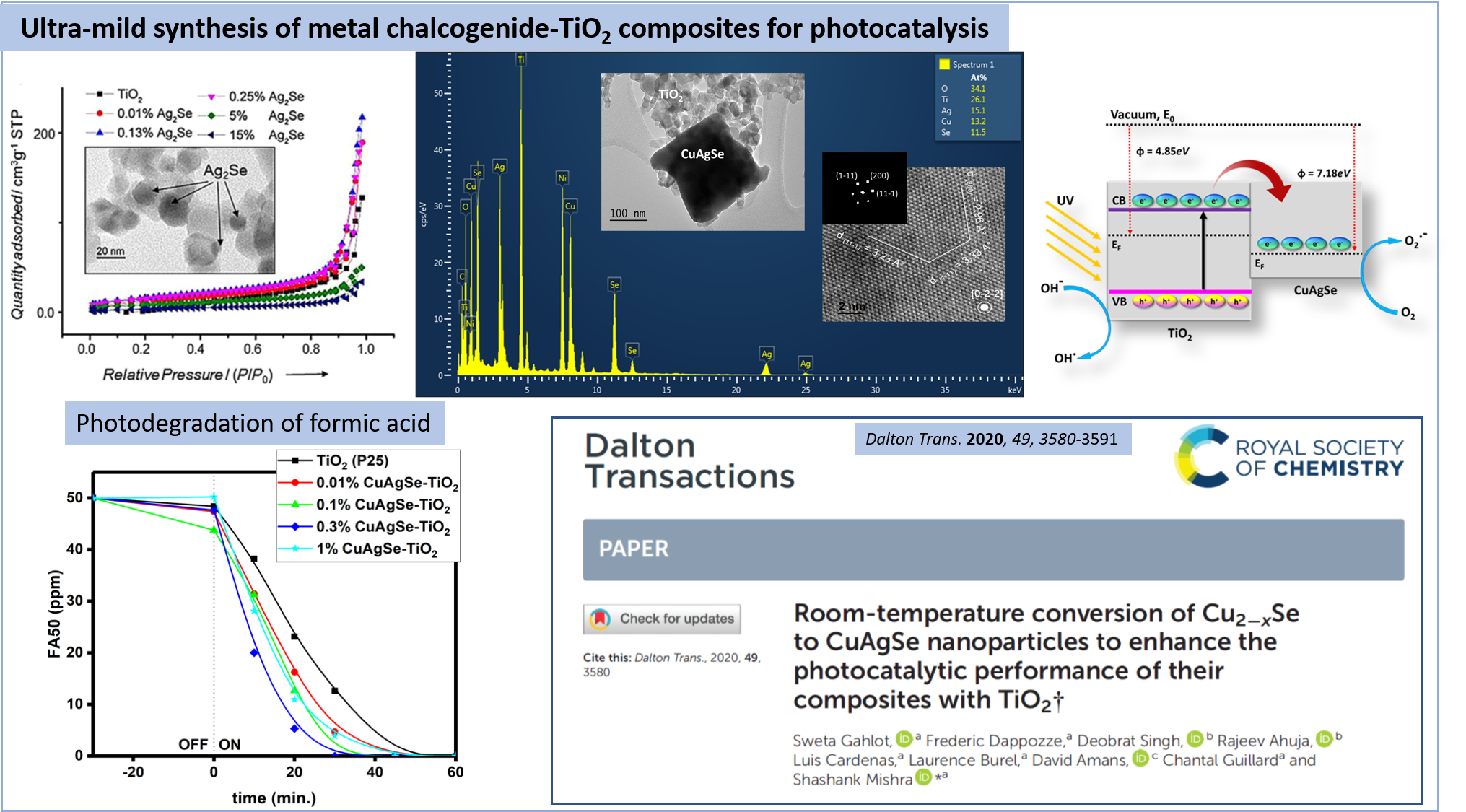
Room-temperature conversion of Cu2-xSe to CuAgSe nanoparticles to enhance the photocatalytic performance of their composites with TiO2
Dalton transactions, 2020, 49, pp. 3580-3591
Purohit B., Guyot Y., Amans D., Joubert M., Mahler B.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Dujardin C., Ledoux G.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Dujardin C., Ledoux G.
Multicolor Solar Absorption as a Synergetic UV Upconversion Enhancement Mechanism in LiYF4:Yb3+,Tm3+ Nanocrystals
Acs photonics, 2019, 6, pp. 3126-3131
Bhattacharyya B., Biswas J.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Gogoi N.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Gogoi N.
Rapid Suzuki-Miyaura cross-coupling reaction catalyzed by zirconium carboxyphosphonate supported mixed valent Pd(0)/Pd(II) catalyst
Applied organometallic chemistry, 2019, 33, p.
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,  Franck Morfin Personnel technique et administratif 445 331 (Standard + 33 [0] 472 445 300) P406 Morfin F., Mendez V., Swamy P.,
Franck Morfin Personnel technique et administratif 445 331 (Standard + 33 [0] 472 445 300) P406 Morfin F., Mendez V., Swamy P.,  Jean-Luc Rousset Chercheur 445 434 (Standard + 33 [0] 472 445 300) P404 Rousset J.-L., Daniele S.
Jean-Luc Rousset Chercheur 445 434 (Standard + 33 [0] 472 445 300) P404 Rousset J.-L., Daniele S.
Nanometric NaYF4 as an Unconventional Support for Gold Catalysts for Oxidation Reactions
Acs omega, 2019, 4, pp. 5852-5861
Gahlot S., Jeanneau E.,  Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,
Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,  Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C.,
Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C.,  Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S.
Precursor-mediated synthesis of Cu2-xSe nanoparticles and their composites with TiO2 for improved photocatalysis
Dalton transactions, 2018, 47, pp. 8897-8905
Soussi K.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Mantoux A., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Mantoux A., Daniele S.
Synthesis, characterization and thermal transport properties of heteroleptic N-alkyl triazenide complexes of titanium(IV) and niobium(V)
Polyhedron, 2018, 152, pp. 84-89
Borah S.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.,  Luis Cardenas Chercheur 445 314 (Standard + 33 [0] 472 445 300) P205 Cardenas L., Gogoi N.
Luis Cardenas Chercheur 445 314 (Standard + 33 [0] 472 445 300) P205 Cardenas L., Gogoi N.
Pd Nanoparticles Dispersed on Zr-IV Organophosphonate: A Robust and Reusable Catalyst for Suzuki-Miyaura Cross-Coupling Reactions
European journal of inorganic chemistry, 2018, pp. 751-758
Ledoux G., Amans D., Joubert M., Mahler B.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Dujardin C.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Dujardin C.
Modeling Energy Migration for Upconversion Materials
Journal of physical chemistry c, 2018, p.
Aviziotis I., Duguet T., Soussi K., Heggen M., Lafont M.,  Franck Morfin Personnel technique et administratif 445 331 (Standard + 33 [0] 472 445 300) P406 Morfin F.,
Franck Morfin Personnel technique et administratif 445 331 (Standard + 33 [0] 472 445 300) P406 Morfin F.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Boudouvis A., Vahlas C.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Boudouvis A., Vahlas C.
Chemical Vapor Deposition of Al13Fe4 Highly Selective Catalytic Films for the Semi-Hydrogenation of Acetylene
Physica status solidi a-applications and materials science, 2018, 215, p.
Fang W,  Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,
Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,  Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C., Zhou Y, Xing M,
Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C., Zhou Y, Xing M,  Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S., Daniele S, Zhang J
Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S., Daniele S, Zhang J
Zn-Assisted TiO2-x Photocatalyst with Efficient Charge Separation for Enhanced Photocatalytic Activities
Journal of physical chemistry c, 2017, 121, pp. 17068-17076
Soussi K.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Millet J. M. M., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Millet J. M. M., Daniele S.
Asymmetrically substituted triazenes as poor electron donor ligands in the precursor chemistry of iron(II) for iron-based metallic and intermetallic nanocrystals
DALTON TRANSACTIONS, 2017, 46(38), pp. 13055-13064
Borah S., Bhattacharyya B., Deka J., Borah A., Devi A., Deka D.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Raidongia K., Gogoi N.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Raidongia K., Gogoi N.
Enhanced catalytic activity and near room temperature gas sensing properties of SnO2 nanoclusters@mesoporous Sn(IV) organophosphonate composite
Dalton Transactions, 2017, 46, pp. 8664-8672
Fang W. Z., Khrouz L., Zhou Y., Shen B., Dong C. Y., Xing M. Y.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Zhang J. L.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Zhang J. L.
Reduced {001}-TiO2-x photocatalysts: noble-metal-free CO2 photoreduction for selective CH4 evolution
Physical Chemistry Chemical Physics, 2017, 21, pp. 13875-13881
 Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S., Du D., Jeanneau E.,
Shashank Mishra University researcher 445 360 (Switchboard + 33 [0] 472 445 300) P402 Mishra S., Du D., Jeanneau E.,  Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,
Frederic Dappozze Technical and administrative staff 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Dappozze F.,  Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C., Zhang J., Daniele S.
Chantal Guillard Researcher 445 316 (Switchboard + 33 [0] 472 445 300) P1211 Guillard C., Zhang J., Daniele S.
A Facile Molecular Precursor-based Synthesis of Ag2 Se Nanoparticles and Its Composites with TiO2 for Enhanced Photocatalytic Activity
Chemistry : an Asian Journal, 2016, 11, pp. 1658-63
Sharma Nandu Bala, Shahani J., Ghadwal Rajendra Singh, Singh A., Jeanneau E.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Homometallic glycolates containing hydroxyl functionality for anchoring another metal: synthesis and characterization of heterometallic alkoxide-glycolates of Ti and Zr incorporating Al and Nb
Journal of Coordination Chemistry, 2016, 69, pp. 135-148
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Rolland M., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Rolland M., Daniele S.
Structural isomers of iron(III) N-methyl diethanolaminate as sol-gel precursors for iron-based oxide nanomaterials
RSC Advances, 2016, 6, pp. 1738-1743
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S.
Metal-Organic Derivatives with Fluorinated Ligands as Precursors for Inorganic Nanomaterials
Chemical reviews, 2015, 115, pp. 8379-448
Ayadi H., Fang W.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Zhang J., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Zhang J., Daniele S.
Influence of Na+ ion doping on the phase change and upconversion emissions of the GdF3: Yb3+, Tm3+ nanocrystals obtained from the designed molecular precursors
RSC Advances, 2015, 5, pp. 100535-100545
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Mangematin S., Chermette H., Kalhor Mahboubeh Poor, Bonnefont G., Fantozzi G., Le Floch Sylvie, Pailhes S., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Mangematin S., Chermette H., Kalhor Mahboubeh Poor, Bonnefont G., Fantozzi G., Le Floch Sylvie, Pailhes S., Daniele S.
A convenient and quantitative route to Sn(IV)-M M = Ti(IV)\, Nb(V)\, Ta(V) heterobimetallic precursors for dense mixed-metal oxide ceramics
Dalton Transactions, 2015, 44, pp. 6848-6862
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Daniele S.
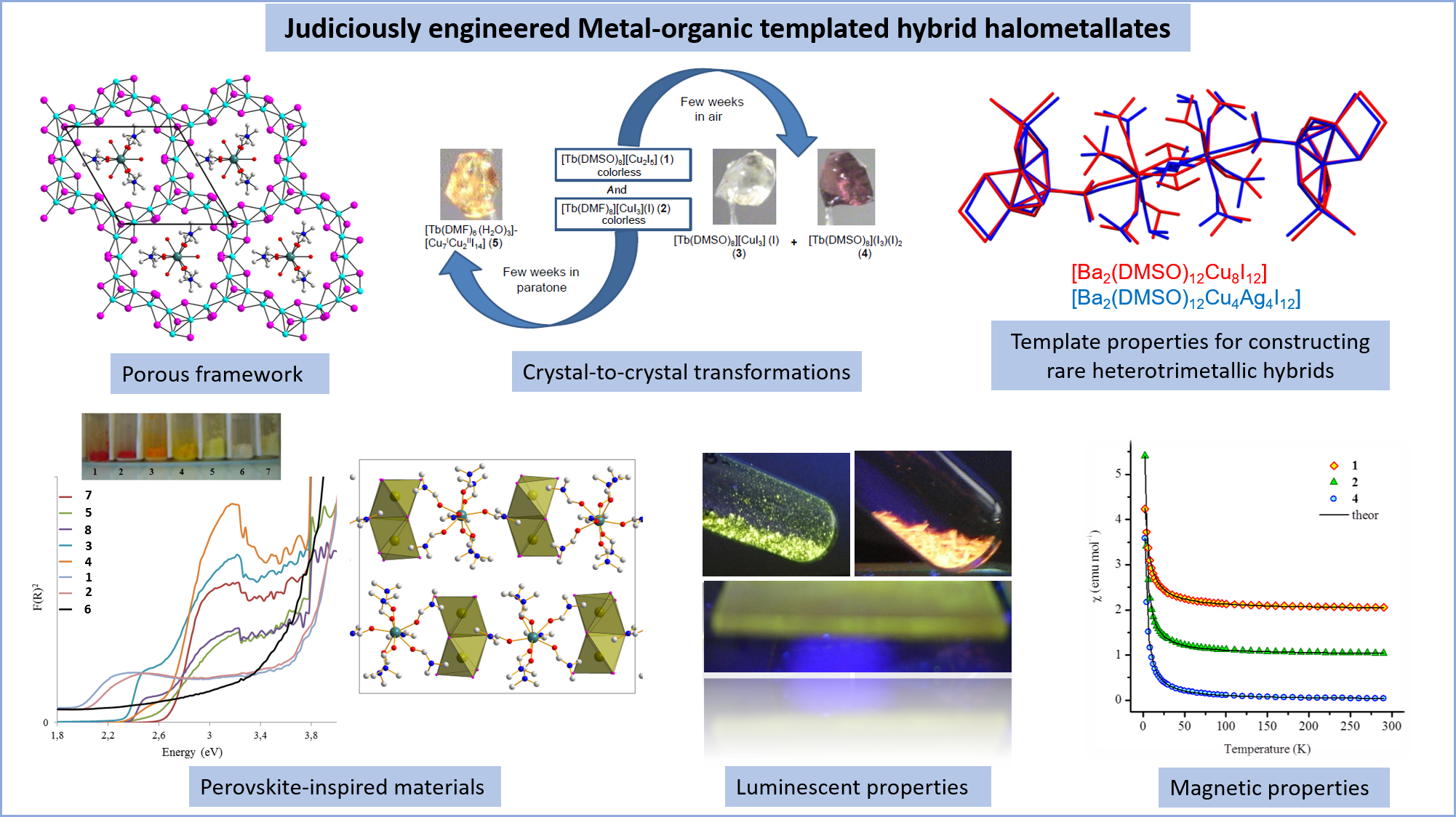
Novel Barium-Organic Incorporated lodometalates: Do They Have Template Properties for Constructing Rare Heterotrimetallic Hybrids?
Inorganic Chemistry, 2014, 53, pp. 11721-11731
Chen Y.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Ledoux G., Jeanneau E., Daniel Marlã¨ne, Zhang J. L., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Ledoux G., Jeanneau E., Daniel Marlã¨ne, Zhang J. L., Daniele S.
Direct Synthesis of Hexagonal NaGdF4 Nanocrystals from a Single-Source Precursor: Upconverting NaGdF4:Yb3+\, Tm3+ and Its Composites with TiO2 for Near-IR-Driven Photocatalysis
Chemistry - An Asian Journal, 2014, 9, pp. 2415-2421
Machon D., Piot L., Hapiuk D., Masenelli B., Demoisson F., Piolet R., Ariane M.,  Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Hosni M., Jouini N., Farhat S., Meelinon P.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Hosni M., Jouini N., Farhat S., Meelinon P.
Thermodynamics of Nanoparticles: Experimental Protocol Based on a Comprehensive Ginzburg-Landau Interpretation
Nano Letters, 2014, 14, pp. 269-276
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Bulin A. L., Ledoux G., Jouguet B., Amans D., Belsky A., Daniele S., Dujardin C.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Bulin A. L., Ledoux G., Jouguet B., Amans D., Belsky A., Daniele S., Dujardin C.
A molecular precursor approach to monodisperse scintillating CeF3 nanocrystals
Dalton Transactions, 2013, 42, pp. 12633-12643
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Mendez V., Jeanneau E., Caps V., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Mendez V., Jeanneau E., Caps V., Daniele S.
A Single Source Precursor Route to Group 13 Homo- and Heterometallic Oxides as Highly Active Supports for Gold-Catalyzed Aerobic Epoxidation of trans-Stilbene
European Journal of Inorganic Chemistry, 2013, pp. 500-510
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Iasco O., Ledoux G., Luneau D., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Iasco O., Ledoux G., Luneau D., Daniele S.
Heterometallic, Hybrid, Heavy Main-Group Iodometallates Containing Lanthanide Complexes: Template Synthesis, Structures, Thermal, Optical, Luminescent and Magnetic Properties
European Journal of Inorganic Chemistry, 2012, xx, pp. 2749-2758
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Daniele S.
Solid-state structural transformations in metal organic-inorganic hybrids constructed from terbium(III) complexes and iodocuprate clusters
CrystEngComm, 2012, 14, pp. 3894-3901
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Ledoux G., Jeanneau E., Daniele S., Joubert M. F.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Ledoux G., Jeanneau E., Daniele S., Joubert M. F.
Novel heterometal-organic complexes as first single source precursors for up-converting NaY(Ln)F-4 (Ln = Yb, Er, Tm) nanomaterials
Dalton Transactions, 2012, 41, pp. 1490-1502
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Berger M. H., Hochepied J. F., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Berger M. H., Hochepied J. F., Daniele S.
Novel Heteroleptic Heterobimetallic Alkoxide Complexes as Facile Single-Source Precursors for Ta5+ Doped TiO2-SnO2 Nanoparticles
Inorganic Chemistry, 2010, 49, pp. 11184-11189
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Mendez V.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Mendez V.
Aminoalkoxo-supported heteroleptic hexanuclear gallium(III) wheel as a synthon for group 13 heterometallics: A rare sol-gel precursor for mixed Al-Ga oxide as support for gold catalysts
Dalton Transactions, 2010, 39, pp. 7440-7443
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Ledoux G., Jeanneau E., Joubert M. F.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Ledoux G., Jeanneau E., Joubert M. F.
Heterometallic Na-Y(Ln) trifluoroacetate diglyme complexes as novel single-source precursors for upconverting NaYF4 nanocrystals co-doped with Yb and Er/Tm ions
Chemical Communications, 2010, 46, pp. 3756-3758
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S.
Dimethyl selenide complexes of copper, gallium and indium halides as potential precursors for selenium-containing chalcopyrite semiconducting materials
Polyhedron, 2010, 29, pp. 500-506
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Petit S., Jeanneau E., Rolland M.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Petit S., Jeanneau E., Rolland M.
Homoleptic gallium(III) and indium(III) aminoalkoxides as precursors for sol-gel routes to metal oxide nanomaterials
Dalton Transactions, 2009, pp. 2569-2577
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Daniele S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Ledoux G., Daniele S.
Lanthanide complexes in hybrid halometallate materials: interconversion between a novel 2D microporous framework and a 1D zigzag chain structure of iodoargentates templated by octakis-solvated terbium(III) cation
Dalton Transactions, 2009, pp. 4954-4961
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Hubert-Pfalzgraf L. G., Daniele S., Rolland M., Jeanneau E., Jouguet B.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Hubert-Pfalzgraf L. G., Daniele S., Rolland M., Jeanneau E., Jouguet B.
Thermal dehydration of Y(TFA)(3)(H2O)(3): Synthesis and molecular structures of Y(mu, eta(1):eta(1)-TFA)(3)(THF)(H2O) (1 infinity) center dot THF and Y-4(mu(3)-OH)(4)(mu, eta(1):eta(1)-TFA)(6)(eta(1)-TFA) (eta(2)-TFA)(THF)(3)(DMSO)(H2O) center dot 6THF (TFA = trifluoroacetate)
Inorganic Chemistry Communications, 2009, 12, pp. 97-100
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S.
Anhydrous scandium, yttrium, lanthanide and actinide halide complexes with neutral oxygen and nitrogen donor ligands
Coordination Chemistry Reviews, 2008, 252, pp. 1996-2025
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Chermette H., Daniele S., Hubert-Pfalzgraf L. G.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Chermette H., Daniele S., Hubert-Pfalzgraf L. G.
Crystal-to-crystal transformations in heterometallic yttrium(III)-copper(I) iodide derivatives in a confined solvent-free environment: Influence of solvated yttrium cations on the nuclearity and dimensionality of iodocuprate clusters
Dalton Transactions, 2008, pp. 620-630
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Hubert-Pfalzgraf L. G.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Hubert-Pfalzgraf L. G.
Rare example of a polynuclear heterometallic yttrium(III)-copper(I) iodide cluster with a Y-6(mu(6)-O)(mu(3)-OH)(8) (8+) core structure showing single crystal-to-single crystal transformation
CrystEngComm, 2008, 10, pp. 814-816
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Ledoux G.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Ledoux G.
Reactions of metal iodides as a simple route to heterometallics: synthesis, structural transformations, thermal and luminescent properties of novel hybrid iodoargentate derivatives templated by YL8 (3+) or YL7 (3+) cations (L = DMF or DMSO)
Dalton Transactions, 2008, pp. 6296-6304
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Ledoux G., Swamy P. N.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Jeanneau E., Daniele S., Ledoux G., Swamy P. N.
Solid- and Solution Phase Transformations in Novel Hybrid Iodoplumbate Derivatives Templated by Solvated Yttrium Complexes
Inorganic Chemistry, 2008, 47, pp. 9333-9343
Shashank Mishra, Jianyong Zhang, Liliane G. Hubert-Pfalzgraf, Dominique Luneau, Erwann Jeanneau
The Interplay between Yttrium and Barium or Copper Trifluoroacetates and N-Methyldiethanolamine: Synthesis of a Heterometallic Y3
Cu Trifluoroacetate Complex and a Homometallic Ba-TFA 1D Polymer
European Journal of Inorganic Chemistry, 2007, pp. 602-608
Shashank Mishra, Liliane G. Hubert Pfalzgraf, Erwann Jeanneau, Henry Chermette
From discrete [Y(DMF)8][Cu4(µ3-I)2(µ-I)3I2] ion pairs to extended [Y(DMF)6(H2O)2][Cu7(µ4-I)3(µ3-I)2(µ-I)4(I)]1∞ and [Y(DMF)6(H2O)3][CuI7CuII2(µ3-I)8(µ-I)6]2∞ arrays by H-bond templating in a confined solvent-free environment
Dalton Transactions, 2007, pp. 410-413
Shashank Mishra, Liliane G. Hubert-Pfalzgraf, Marc Rolland, Henry Chermette
Activation of dioxygen by an yttrium iodide adduct: First example of an yttrium superoxide displaying also a rare 1,1-bridging mode of the superoxide ligand
Inorganic Chemistry Communications, 2007, 10(1), pp. 15-19
Shashank Mishra, Liliane G. Hubert Pfalzgraf, Erwann Jeanneau
Redistribution reactions of heteroleptic barium iodide derivatives: Synthesis and structures of trans-BaI2(DME)(triglyme), cis-BaI2(DME)(tetraglyme) and [Ba(tetraglyme)2]I2 · C7H8
Polyhedron, 2007, 26, p. 66
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Hubert-Pfalzgraf L. G.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Hubert-Pfalzgraf L. G.
Metal 2-ethylhexanoates and related compounds as useful precursors in materials science
Chemical Society Reviews, 2007, 36, pp. 1770-1787
 Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Hubert-Pfalzgraf L. G., Jeanneau E.
Shashank Mishra Enseignant-chercheur 445 360 (Standard + 33 [0] 472 445 300) P402 Mishra S., Daniele S., Hubert-Pfalzgraf L. G., Jeanneau E.
Hydrolysis of a (2-Propanol)yttrium Triiodide Complex in the Presence of Glymes: Synthesis and X-ray Structures of Hydroxo-Bridged Dinuclear Yttrium Complexes and Their Applications in Materials Science
European Journal of Inorganic Chemistry, 2007, 15, pp. 2208-2215