Rafael Garduño‐ibarra I., El Hadj G.,  Zhigang Yan Student or nonpermanent staff 431 587 (Switchboard + 33 [0] 472 445 300) C-15002 Yan Z.,
Zhigang Yan Student or nonpermanent staff 431 587 (Switchboard + 33 [0] 472 445 300) C-15002 Yan Z.,  Anne Bonhommé Technical and administrative staff 445 477 (Switchboard + 33 [0] 472 445 300) P1104 Bonhommé A., Aouines M., S. Prévot M.,
Anne Bonhommé Technical and administrative staff 445 477 (Switchboard + 33 [0] 472 445 300) P1104 Bonhommé A., Aouines M., S. Prévot M.,  Laurent Djakovitch Researcher 445 381 (Switchboard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Researcher 445 381 (Switchboard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Philippe Vernoux Researcher 431 587 (Switchboard + 33 [0] 472 445 300) C15.009 Vernoux P., González‐cobos J.,
Philippe Vernoux Researcher 431 587 (Switchboard + 33 [0] 472 445 300) C15.009 Vernoux P., González‐cobos J.,  Adel Mesbah Researcher 445 489 (Switchboard + 33 [0] 472 445 300) P-1308 Mesbah A.
Adel Mesbah Researcher 445 489 (Switchboard + 33 [0] 472 445 300) P-1308 Mesbah A.
Facile Controlling of Active Sites on NiOOH from Nickel Hydroxy‐Muconate for Oxygen Evolution Reaction
ChemCatChem, 2025, pp. -
 Ajay Tomer Personnel étudiant ou non permanent 445 300 (Standard + 33 [0] 472 445 300) P-213 Tomer A.,
Ajay Tomer Personnel étudiant ou non permanent 445 300 (Standard + 33 [0] 472 445 300) P-213 Tomer A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Noemie Perret Chercheur 445 446 (Standard + 33 [0] 472 445 300) P211 Perret N.
Noemie Perret Chercheur 445 446 (Standard + 33 [0] 472 445 300) P211 Perret N.
Evaluation of Ni/TiO2 catalysts in the semi-hydrogenation of alkynols under mild conditions in water
GREEN CHEMISTRY, 2025, p.
Huerta A.,  Mahmud Beji Ahmed Personnel étudiant ou non permanent 445 300 (Standard + 33 [0] 472 445 300) P-1306 Ahmed M. B.,
Mahmud Beji Ahmed Personnel étudiant ou non permanent 445 300 (Standard + 33 [0] 472 445 300) P-1306 Ahmed M. B.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Adel Mesbah Chercheur 445 489 (Standard + 33 [0] 472 445 300) P-1308 Mesbah A., Loiseau T., Duval S.
Adel Mesbah Chercheur 445 489 (Standard + 33 [0] 472 445 300) P-1308 Mesbah A., Loiseau T., Duval S.
Polynuclear tantalum(v) coordination complexes: from dinuclear {Ta2O} to octanuclear {Ta8O12} oxo species connected through aryl monotopic carboxylate linkers
CRYSTENGCOMM, 2025, p.
Manas A.,  Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Catalytic Oxidation of Kraft Lignin in a Trickle-Bed Continuous Reactor
CHEMPLUSCHEM, 2025, p.
Reynoso A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Noemie Perret Chercheur 445 446 (Standard + 33 [0] 472 445 300) P211 Perret N.
Noemie Perret Chercheur 445 446 (Standard + 33 [0] 472 445 300) P211 Perret N.
Acceptorless Dehydrogenation of Alcohols and Polyols Over Cu-Based Catalysts Prepared by NaBH4 Reduction
CHEMCATCHEM, 2024, p.
Hernandez-Manas A., Martinez-Martin A., Madignier J.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P., Bertaud F.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P., Bertaud F.,  Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Copper catalyzed alkaline aerobic lignin depolymerization: effect of botanical origin and industrial extraction process on reactivity supported through characterization
RSC SUSTAINABILITY, 2024, p.
 Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L., Chaussard N., Manas A., Boyron O., Taam M., Bertaud F.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L., Chaussard N., Manas A., Boyron O., Taam M., Bertaud F.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
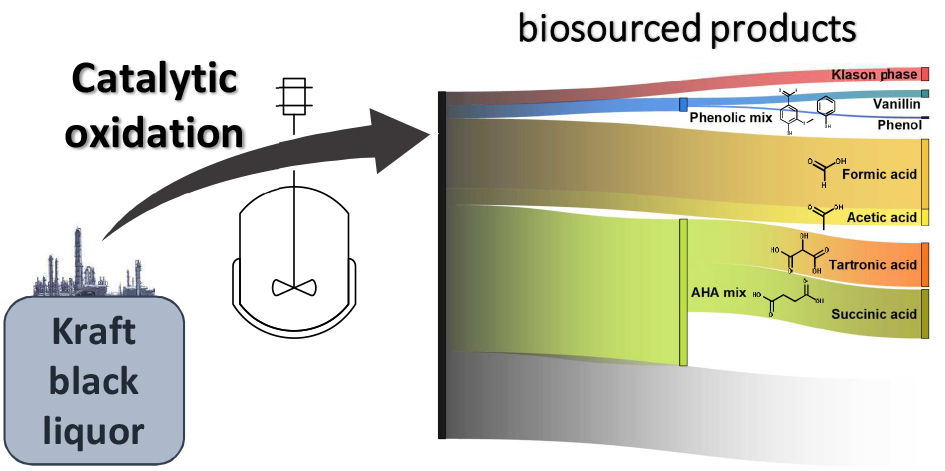
Potential of catalytic oxidation of kraft black liquor for the production of biosourced compounds
GREEN CHEMISTRY, 2023, p.
Manas A.,  Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Lignin Catalytic Oxidation by CuO/TiO2: Role of Catalyst in Phenolics Formation
WASTE AND BIOMASS VALORIZATION, 2023, p.
Manas A., Chaussard N., Bertaud F.,  Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
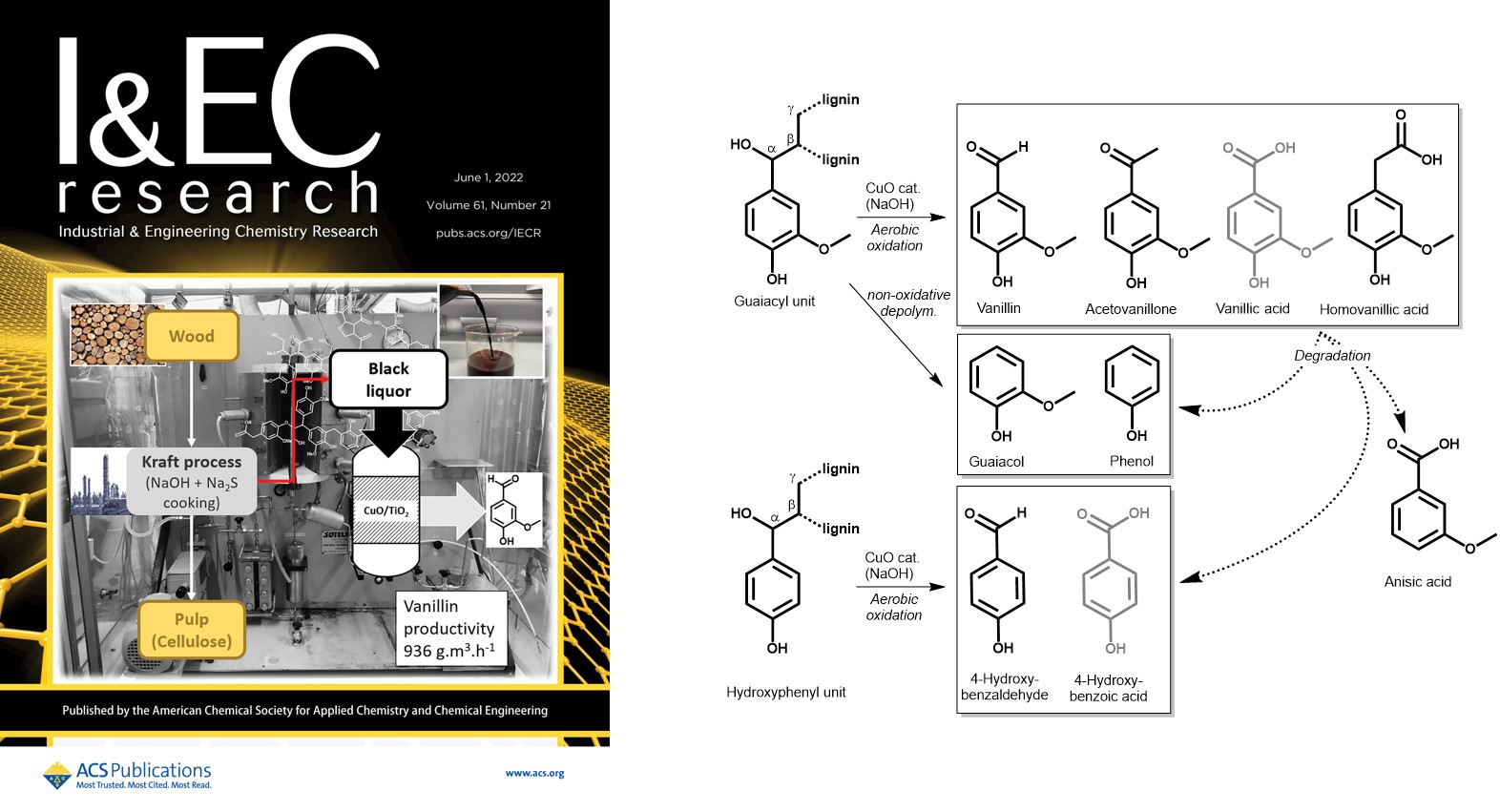
Production of Phenolic Compounds from Catalytic Oxidation of Kraft Black Liquor in a Continuous Reactor
INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, 2022, 61(21), pp. 7430-7437
 Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,
Nadine Essayem Chercheur 445 315 (Standard + 33 [0] 472 445 300) P1314 Essayem N.,  Marion Eternot Personnel technique et administratif 445 375 (Standard + 33 [0] 472 445 300) P1303 Eternot M.,
Marion Eternot Personnel technique et administratif 445 375 (Standard + 33 [0] 472 445 300) P1303 Eternot M.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
A Landscape of Lignocellulosic Biopolymer Transformations into Valuable Molecules by Heterogeneous Catalysis in C'Durable Team at IRCELYON
MOLECULES, 2021, 26(22), p.
Lutoshkin M.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Malyar Y., Kuznetsov B.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Malyar Y., Kuznetsov B.
Composition and Structure of Ethanol-Lignins of Coniferous and Deciduous Wood and Products of their Catalytic Telomerization with 1,3-Butadiene
JOURNAL OF SIBERIAN FEDERAL UNIVERSITY-CHEMISTRY, 2021, 14(4), pp. 539-551
Bourbiaux D., Xu Y., Burel L., Goc F.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P., Philippe R., Aubert G., Aymonier C.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P., Philippe R., Aubert G., Aymonier C.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Investigating (pseudo)-heterogeneous Pd-catalysts for kraft lignin depolymerization under mild aqueous basic conditions
Catalysts, 2021, 11(11), p. 1311
De Tovar J.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Heterogenization of Pd(II) complexes as catalysts for the Suzuki-Miyaura reaction
APPLIED CATALYSIS A-GENERAL, 2021, 627, p.
Kuznetsov B., Sharypov V., Baryshnikov S., Miroshnikova A., Taran O., Yakovlev V., Lavrenov A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium
CATALYSIS TODAY, 2021, 379, pp. 114-123
Bourbiaux D., Pu J.,  Franck Rataboul Researcher 445 338 (Switchboard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Researcher 445 338 (Switchboard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Researcher 445 381 (Switchboard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Researcher 445 381 (Switchboard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Christophe Geantet Researcher 445 336 (Switchboard + 33 [0] 472 445 300) P207 Geantet C.,
Christophe Geantet Researcher 445 336 (Switchboard + 33 [0] 472 445 300) P207 Geantet C.,  Dorothee Laurenti Researcher 445 327 (Switchboard + 33 [0] 472 445 300) P310 Laurenti D.
Dorothee Laurenti Researcher 445 327 (Switchboard + 33 [0] 472 445 300) P310 Laurenti D.
Reductive or oxidative catalytic lignin depolymerization: An overview of recent advances
CATALYSIS TODAY, 2021, 373, pp. 24-37
Sebhat W., El Roz A.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P.,  Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Catalytic Liquefaction of Kraft Lignin with Solvothermal Approach
CATALYSTS, 2021, 11(8), p.
Kuznetsov B., Sudakova I., Garyntseva N., Tarabanko V., Yatsenkova O.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Processes of catalytic oxidation for the production of chemicals from softwood biomass
CATALYSIS TODAY, 2021, 375, pp. 132-144
De Tovar J.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
From the grafting of NHC-based Pd(II) complexes onto TiO2 to the in situ generation of Mott-Schottky heterojunctions: The boosting effect in the Suzuki-Miyaura reaction. Do the evolved Pd NPs act as reservoirs?
JOURNAL OF CATALYSIS, 2021, 398, pp. 133-147
Cabral Almada C., Kazachenko A.,  Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P., Da Silva Perez D., Kuznetsov B.,
Pascal Fongarland Enseignant-chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Fongarland P., Da Silva Perez D., Kuznetsov B.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization
CATALYSTS, 2021, 11(4), p.
Bourbiaux D., Mangematin S.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Selective Aerobic Oxidation of Benzyl Alcohols with Palladium(0) Nanoparticles Suspension in Water
CATALYSIS LETTERS, 2021, p.
Bourouina A., Oswald A., Lido V., Dong L.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., De Bellefon C.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., De Bellefon C.,  Valerie Meille Chercheur 432 625 (Standard + 33 [0] 472 445 300) C-15.011 Meille V.
Valerie Meille Chercheur 432 625 (Standard + 33 [0] 472 445 300) C-15.011 Meille V.
Kinetic Study of the Herrmann–Beller Palladacycle-Catalyzed Suzuki–Miyaura Coupling of 4-Iodoacetophenone and Phenylboronic Acid
Catalysts, 2020, 10, p. 989
De Tovar J.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Insights into the Suzuki-Miyaura Reaction Catalyzed by Novel Pd-Carbene Complexes. Are Palladium-Tetra- carbene Entities the Key Active Species?
CHEMCATCHEM, 2020, 12(22), pp. 5797-5808
Almada C., Kazachenko A., Fongarland P., Perez D., Kuznetsov B.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Oxidative depolymerization of lignins for producing aromatics: variation of botanical origin and extraction methods
Biomass conversion and biorefinery, 2020, p.
Sebhat W., El-Roz A., Crepet A., Ladaviere C., Perez D., Mangematin S., Almada C.,  Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,
Lea Vilcocq Chercheur 445 300 (Standard + 33 [0] 472 445 300) C-13.010 Vilcocq L.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Fongarland P.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Fongarland P.
Comparative study of solvolysis of technical lignins in flow reactor
Biomass conversion and biorefinery, 2020, 10, pp. 351-366
Kuznetsov B., Sudakova I., Garyntseva N., Tarabanko V., Chesnokov N.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Kinetic Studies and Optimization of Heterogeneous Catalytic Oxidation Processes for the Green Biorefinery of Wood
Topics in catalysis, 2020, 63, pp. 229-242
Zahreddine W., Karame I.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
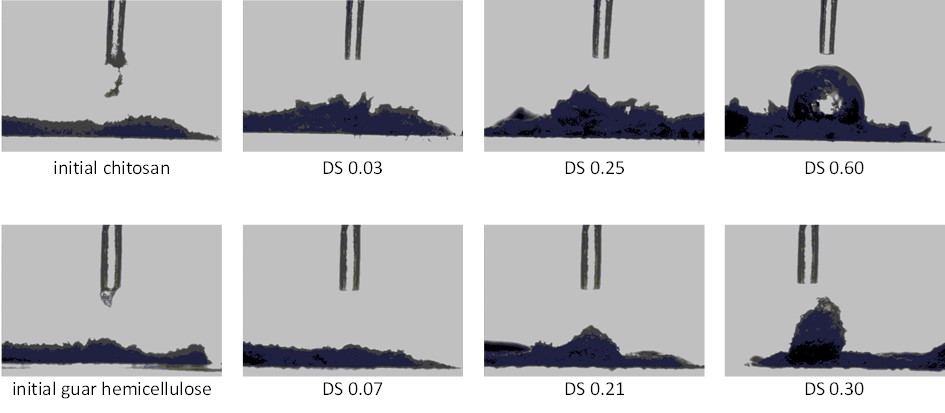
First study on telomerization of chitosan and guar hemicellulose with butadiene: Influence of reaction parameters on the substitution degree of the biopolymers
Molecular catalysis, 2020, 483, p. 110706
Baryshnikov S., Miroshnikova A., Kazachenko A., Malyar Y., Taran O., Lavrenov A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Kuznetsov B.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Kuznetsov B.
Hydrogenation of Abies Wood and Ethanol Lignin by Hydrogen in Supercritical Ethanol in the Presence of Bifunctional Catalyst Pt/ZrO2
Journal of siberian federal university-chemistry, 2019, 12, pp. 550-561
Kuznetsov B., Sudakova I., Garyntseva N., Kondrasenko A., Pestunov A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catalytic peroxide fractionation processes for the green biorefinery of wood
Reaction kinetics mechanisms and catalysis, 2019, 126, pp. 717-735
Marcel R., Durillon T.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Fache F.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Fache F.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
First Example of the Use of Biosourced Alkyl Levulinates as Solvents for Synthetic Chemistry: Application to the Heterogeneously Catalyzed Heck Coupling
Chemistryselect, 2019, 4, pp. 3329-3333
Levdansky A., Vasilyeva N., Maximov N., Karacharov A., Levdansky V., Skvortsova G., Kuznetsov B.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Synthesis and Study of Copper-Containing Polymers of Microcrystalline Cellulose Sulfates from Larch Wood
Russian journal of bioorganic chemistry, 2018, 44, pp. 834-838
Kuznetsov B., Sudakova I., Yatsenkova O., Garyntseva N.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Optimizing Single-Stage Processes of Microcrystalline Cellulose Production via the Peroxide Delignification of Wood in the Presence of a Titania Catalyst
Catalysis in industry, 2018, 10, pp. 360-367
Kuznetsov B., Sudakova I., Garyntseva N., Levdansky V., Ivanchenko N., Pestunov A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Green biorefinery of larch wood biomass to obtain the bioactive compounds, functional polymers and nanoporous materials
Wood science and technology, 2018, 52, pp. 1377-1394
Kuznetsov B., Chesnokov N., Sudakova I., Garyntseva N., Kuznetsova S., Malyar Y., Yakovlev V.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Green catalytic processing of native and organosolv lignins
Catalysis today, 2018, 309, pp. 18-30
Rameau N., Russo B., Mangematin S.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Stilbene synthesis through decarboxylative cross-coupling of substituted cinnamic acids with aryl halides
Applied catalysis a-general, 2018, 560, pp. 132-143
Kuznetsov B., Sharypov V., Beregovtsova N., Baryshnikov S., Pestunov A., Vosmerikov A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Thermal conversion of mechanically activated mixtures of aspen wood-zeolite catalysts in a supercritical ethanol
Journal of analytical and applied pyrolysis, 2018, 132, pp. 237-244
Kuznetsov N., Chesnokov N., Garyntseva N., Sudakova I., Pestunov A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Kinetic Study and Optimization of Catalytic Peroxide Delignification of Aspen Wood
Kinetics and catalysis, 2018, 59, pp. 48 - 57
Kuznetsov B., Vasilyeva N., Levdansky A., Maximov N., Kazachenko A., Skvortsova G.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Synthesis and Study of Copper-Containing Polymers Based on Sulfated Arabinogalactan
Russian journal of bioorganic chemistry, 2017, 43, pp. 727-731
Betron C, Bounor-Legare V,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Scalabrino G,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Scalabrino G,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Cassagnau P
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Cassagnau P
Diffusion of modified vegetables oils in thermoplastic polymers
Materials chemistry and physics, 2017, 200, pp. 107-120
Nunes A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Khrouz L., Felpin F. X., Dufaud V.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Khrouz L., Felpin F. X., Dufaud V.
Copper(II)-phenanthroline hybrid material as efficient catalyst for the multicomponent synthesis of 1,2,3-triazoles via sequential azide formation/1,3-dipolar cycloaddition
Molecular Catalysis, 2017, 437, pp. 150-157
Kuznetsov B. N., Sudakova I. G., Garyntseva N. V.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Kinetic studies and optimization of abies wood fractionation by hydrogen peroxide under mild conditions with TiO2 catalyst
Reaction Kinetics Mechanisms and Catalysis, 2017, 120, pp. 81-94
Tarabanko V. E., Kaygorodov K. L., Skiba E. A., Tarabanko N., Chelbina Y. V., Baybakova O. V., Kuznetsov B. N.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Processing pine wood into vanillin and glucose by sequential catalytic oxidation and enzymatic hydrolysis
Journal of Wood Chemistry and Technology, 2017, 37, pp. 43-51
Vasilyeva N., Levdansky A., Kazachenko A., Skvortsova G., Kuznetsov B.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Synthesis of Sulfated Arabinogalactan Derivatives with Histidine and Arginine
Journal of siberian federal university-chemistry, 2016, 9, pp. 318-325
Zahreddine W., Lelong Q., Karame I., Kanj A.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Franck Rataboul Chercheur 445 338 (Standard + 33 [0] 472 445 300) P210 Rataboul F.
Synthesis of terpene derivatives of ethanolamine using telomerization reaction
Tetrahedron Letters, 2016, 57, pp. 452-457
Vasil'Eva N. Yu, Levdansky A. V., Kuznetsov B. N., Skvortsova G. P., Kazachenko A. S.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Sulfation of Arabinogalactan by Sulfamic Acid in Dioxane
Bioorganicheskaya Khimiya / Russian Journal of Bioorganic Chemistry, 2015, 41, pp. 725-731
Ftouni J., Villandier N., Auneau F., Besson M.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
From glycerol to lactic acid under inert conditions in the presence of platinum-based catalysts: The influence of support
Catalysis Today, 2015, 257, pp. 267-273
Kuznetsov B. N., Kuznetsova S. A., Levdansky V. A., Levdansky A. V., Vasil'Eva N. Y., Chesnokov N. V., Ivanchenko N. M.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Optimized methods for obtaining cellulose and cellulose sulfates from birch wood
Wood Science and Technology, 2015, 49, pp. 825-843
Al-Maksoud W., Menuel S., Jahjah M., Monflier E.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Base directed palladium catalysed Heck arylation of acrolein diethyl acetal in water
Applied Catalysis A - General, 2014, 469, pp. 250-258
Auneau F., Noel S., Aubert G., Besson M.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.
On the role of the atmosphere in the catalytic glycerol transformation over iridium-based catalysts
Catalysis Communications, 2011, 16, pp. 144-149
Tarabay J., Al-Maksoud W., Jaber F.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Prakash S.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Prakash S.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Synthesis of diethyl 2-(aryl)vinylphosphonate by the Heck reaction catalysed by supported palladium catalysts
Applied Catalysis A - General, 2010, 388, pp. 124-133
Batail N., Bendjeriou A.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Dufaud V.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Dufaud V.
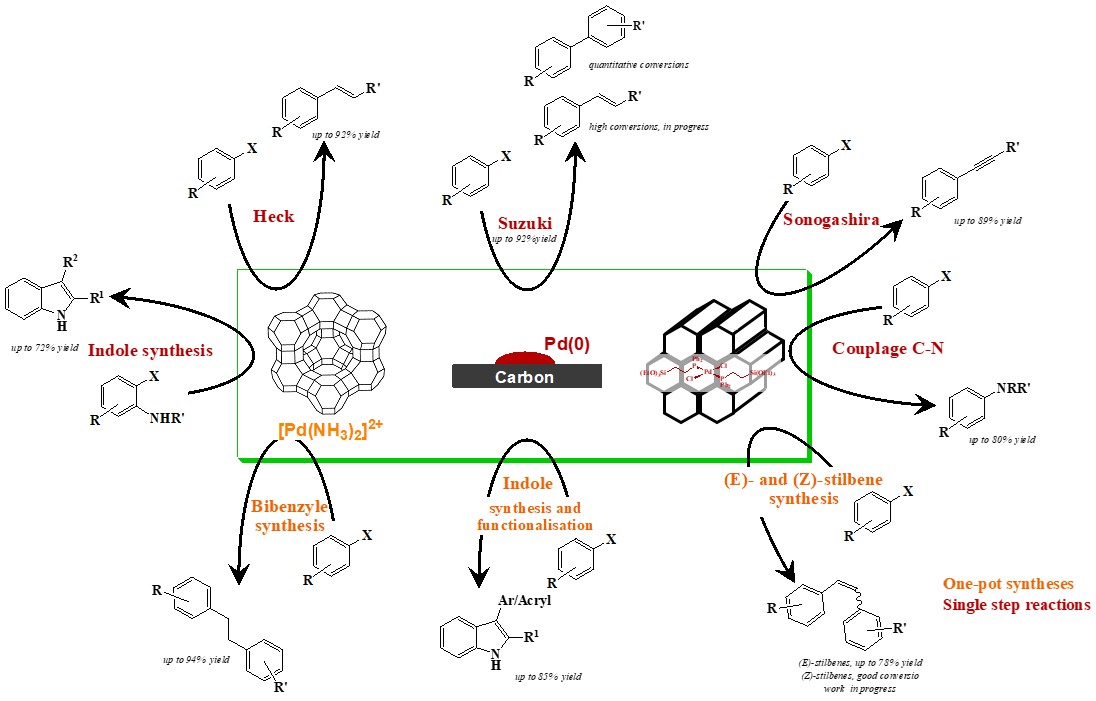
Larock indole synthesis using palladium complexes immobilized onto mesoporous silica
Applied Catalysis A - General, 2010, 388, pp. 179-187
Joucla L., Batail N.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
"On Water" Direct and Site-Selective Pd-Catalysed C-H Arylation of (NH)-Indoles
Advanced Synthesis and Catalysis, 2010, 352, pp. 2929-2936
Joucla L., Cusati G.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
One-Pot Suzuki/Heck Sequence for the Synthesis of (E)-Stilbenes Featuring a Recyclable Silica-Supported Palladium Catalyst via a Multi-Component Reaction in 1,3-Propanediol
Advanced Synthesis and Catalysis, 2010, 352, pp. 1993-2001
Besson M.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Gallezot P.,
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L., Gallezot P.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Salameh A., Vospernik M.
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Salameh A., Vospernik M.
Catalytic Hydrogenolysis of Glycerol
Catalysis of Organice Reactions, Chemical Industries, 2009, 123, pp. 313-318
Debono N.,  Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Jahjah R., Alaaeddine A., Delichere P., Lefebvre F.,
Catherine Pinel Chercheur 445 478 (Standard + 33 [0] 472 445 300) P201 Pinel C., Jahjah R., Alaaeddine A., Delichere P., Lefebvre F.,  Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Laurent Djakovitch Chercheur 445 381 (Standard + 33 [0] 472 445 300) P1403 Djakovitch L.
Asymmetric reduction of ketones with ruthenium-oxazoline based catalysts.
Journal of Molecular Catalysis A: Chemical, 2008, 287, pp. 142-150


 -------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------